Protein assembly is considered to be an essential factor for the development of ordered biological structures, but how about engineering one. This is precisely what scientists at Tokyo Tech have been able to accomplish with protein needles.
Engineered protein needles and their assembly on a mica surface. Scientists have long been attempting to decode the complex sub-structures of proteins. Now, researchers from Tokyo Tech have finally shed light on this front with the investigation of engineered protein self-assembly using protein needles. Image Credit: Takafumi Ueno of Tokyo Institute of Technology.
Through controlling the tip-to-tip interactions of such needles, they enabled their self-assembly into lattice structures, fiber assemblies, and ordered monomeric states, thereby setting the stage for the controlled development of more of such protein architectures.
Proteins are known to be the basic building blocks of the human body. But, their molecular and macroscopic structures seem to be complicated and diverse, with several substructures and folding patterns. Researchers have been attempting to decode these structures for a brief time, and considerable progress has been made as a result of fluorescence microscopy (FM), high-speed AFM (HS-AFM), and atomic force microscopy (AFM).
But, they have not been able to directly note the dynamic motions of proteins during assembly. This is majorly due to the intricate structure of proteins, which are too tiny to be quantified with present methods.
A collaborating team of scientists from Tokyo Institute of Technology (Tokyo Tech), Kyushu University, Nagoya University, and National Institutes of Natural Sciences currently developed a specialized anisotropic protein needle (PN) to help identify the assembly of similarly anisotropic proteins. This offers us clues regarding their microstructure and assembly.
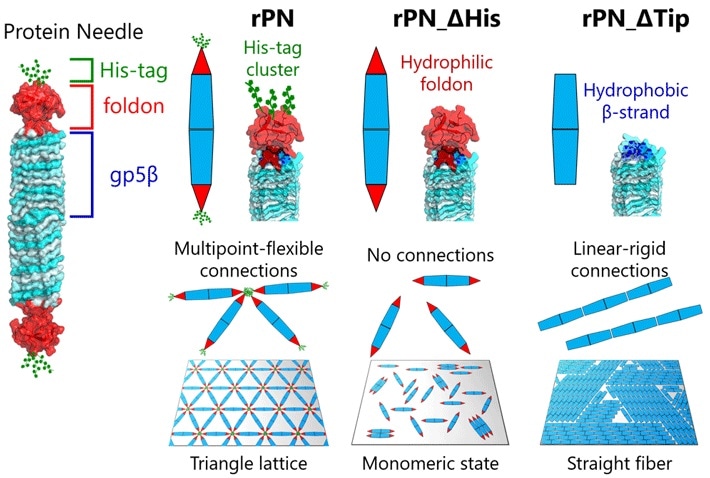
Our PN is a needle-shaped protein composed of the rigid body (β-helix), the terminal cap (foldon), and a binding motif (hexa-histidine tag, His-tag). By modifying these PNs by deleting the His-tag motif and foldon cap, we can produce three different types of PNs.”
Takafumi Ueno, Professor, School of Life Science and Technology, Tokyo Institute of Technology
Ueno added, “This enabled us to regulate and observe different assembly patterns and how they change, giving us clues into the mechanics of different protein-protein interactions that we find in nature.”
The study outcomes have been reported in the journal Small.
In solution, the PNs impulsively develop a highly stable structure measuring a length of around 20 nm and a width of about 3.5 nm. This seems to be small enough to trace the rotational motion of separate molecules yet mechanically strong.
On surfaces, the team noted various kinds of ordered structures as the PNs self-assembled. These structures varied from monomeric states with nematic order (one-dimensional orientation) and triangular lattices to fiber assemblies.
In return, this enabled the team to examine the dynamic processes engaged in protein assembly via a combination of HS-AFM and simulations. The outcomes disclosed that the formation of the triangular lattice structure was directed by the dynamic motions of PN. This adds up to the formation of ordered lattices.
These outcomes have thrilled the scientists who are examining its possible ramifications.
These molecules play such a crucial role in biological systems that understanding their structure would further the field significantly. For instance, we could use this to lay the groundwork for constructing supramolecular structures by designing the dynamic collective motions of proteins. This concept can lead to the engineering of biocompatible sheet materials, targeted drug transports, and even protein-based nano-robots.”
Takafumi Ueno, Professor, School of Life Science and Technology, Tokyo Institute of Technology
Source:
Journal reference:
Kikuchi, K., et al. (2022) Protein Needles Designed to Self-Assemble through Needle Tip Engineering. Small. doi.org/10.1002/smll.202106401.