Scripps Research scientists have found a mechanism that may be able to bypass one of the critical steps in current vaccine production.
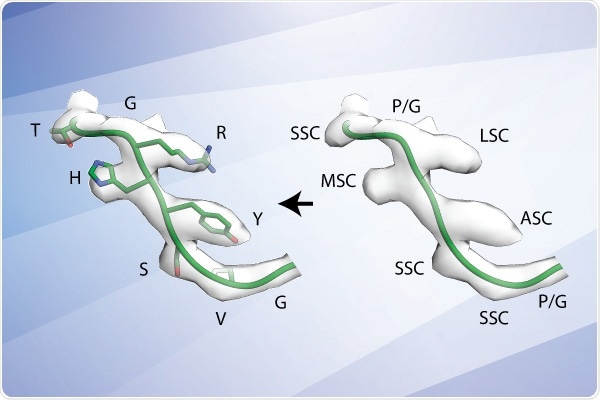
A new method developed at Scripps Research uses cryoEM technology to more quickly identify antibodies for use in vaccine development. In this example, the algorithm screened between approximately 100,000 to 1,000,000 possible antibody sequences from the database to identify the sequence (left) that best matches the antibody observed in their cryoEM images (transparent gray surface). Image Credit: Scripps Research Institute.
The researchers indicated that they could use high-resolution, low-temperature electron microscopy (cryo-EM) to quickly characterize antibodies (stimulated by a vaccine or infection) that bind to the desired target on a virus at an atomic level. Their results will be published in the journal Science Advances on January 19th, 2022.
The COVID-19 pandemic has highlighted the need for robust and rapid vaccine and antiviral technologies. We are optimistic that our new approach will help fill that need by greatly streamlining antibody discovery.”
Andrew Ward PhD, Study Senior Author and Professor, Department of Integrative Structural and Computational Biology, Scripps Research Institute
Aleksandar Antanasijevic PhD, the study’s co-first author, and a postdoctoral research associate, remarked “Traditionally, identifying antibodies that are useful against a virus involves the laborious sorting and testing of antibody-producing B cells to find the right ones—a process that takes months.”
With this new method we can go from blood sample collection from infected or immunized patients to identifying all the elicited antibodies of interest in about ten days.”
Charles Bowman, Study Co-First Author, Staff Scientist, Scripps Research Institute
Recent advancements in cryo-EM, a method that employs a beam of electrons to illuminate and picture objects well below the size of regular light microscopy, helped the researchers achieve their goal.
Researchers utilized high-resolution cryo-EM to identify accurately and quickly where antibodies in rhesus macaque monkeys attach to synthetic versions of the HIV envelope protein that are being produced for possible HIV vaccines in a report published in Nature Communications in August.
The scientists carried this area of research a step further in the current study. They used a computer program called “structure-to-sequence,” which can link antibody structure identified by cryo-EM to the DNA sequence that would make that structure.
Scientists did this by rapidly bulk sequencing the genetic material from antibody-producing B-cells from the monkeys’ lymph nodes to create a database of all the antibody-encoding DNA sequences from the monkeys. The scientists were able to consistently match an antibody of interest in their cryo-EM pictures to a unique antibody identified in the sequence database by applying the algorithm to the cryo-EM data and the antibody sequence library.
The researchers demonstrated that they could validate the precision of the results by utilizing the sequencing data to make copies of that unique, “monoclonal” antibody and confirming using cryo-EM that the antibody attaches in the same way as the originally imaged antibody.
The researchers are now refining their technique to improve its speed and usability, and they are using it to test human antibody responses to experimental HIV vaccines, develop antibody-blocking treatments for autoimmune diseases, and find antibodies that could hit other protein targets on cells therapeutically.
Researchers anticipate that future advances in cryo-EM technology and structure-to-sequence algorithms may enable much faster antibody identification using only high-resolution structural images, without the necessity for B cell DNA sequencing.
This structure-to-sequence approach has a lot of potential in immunology and beyond. We envision being able to use it someday to study protein-to-protein interactions generally, for example, to discover a given protein’s binding partners.”
Aleksandar Antanasijevic PhD, Study Co-First Author and Postdoctoral Research Associate, Scripps Research Institute
New electron microscopy technique could shortcut development of vaccines and antibody therapies
Learn how the new method by scientists at Scripps Research identifies specific antibodies in immune responses to vaccination or infection in fraction of time needed for traditional approach. Video Credit: Scripps Research Institute.
Source:
Journal reference:
Antanasijevic, A., et al. (2022) From structure to sequence: Antibody discovery using cryoEM. Science Advances. doi.org/10.1126/sciadv.abk2039.