Skeletal and heart muscle owe their function as stable biological machines to the outstanding accuracy with which their sarcomeres—the smallest contractile structures—are assembled.
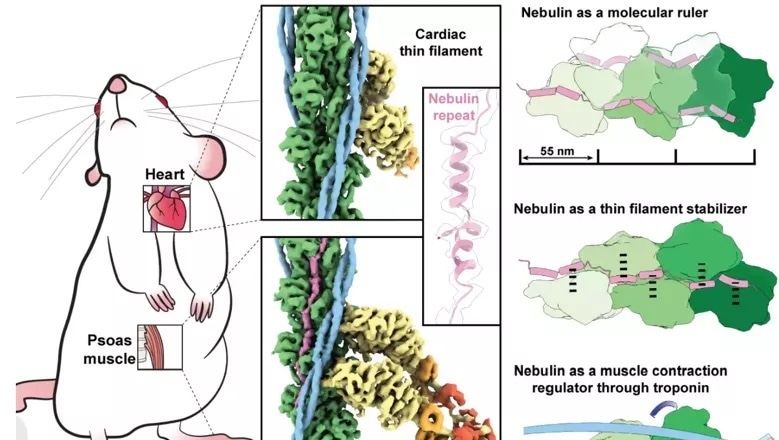
Image Credit: King’s College London
Developing the power to transport blood to all organs and allowing body movements, from breathing to sprint running, these muscles need the synchronized assembly of millions of protein components. These protein compounds are coordinated by the human body’s largest proteins—the giant “rulers,” namely nebulin, titin, and obscurin.
Owing to their huge size, learning these proteins is difficult with technical challenges. Now, researchers have used advanced electron microscopy techniques to acquire the first high-resolution 3D image of nebulin—a giant actin-binding protein that is a vital element of skeletal muscle.
This discovery enables an opportunity to better comprehend the nebulin’s role, as its scope of functioning has remained ambiguous owing to its huge size and the challenge in extracting nebulin from muscle in a native state.
Prof. Mathias Gautel, Head of the School of Basic & Medical Biosciences, Dr Ay Lin Kho, and a team of Max Planck scientists headed by Stefan Raunser, employed electron cryo-tomography to decode the nebulin structure in striking detail. Their results could result in novel therapeutic methods to cure muscular diseases because genetic mutations in nebulin are complemented by a vivid loss in muscle force called nemaline myopathy.
Revealing the exact position of nebulin in the actin filament was an eye-opening insight for us, explaining how it can act as a “counter” for the number of actin molecules. We are excited to now explore even more enigmatic structures of the sarcomere and to ultimately apply this knowledge to the understanding of heart and skeletal muscle diseases.”
Mathias Gautel, Professor and Head, School of Basic & Medical Biosciences, King’s College London
Upon sliding of parallel filaments of the proteins such as myosin and actin, the muscles of skeletons and the heart contract and relax. Nebulin—another long slender protein—which exists only in skeletal muscle, couples up with actin, regulating and stabilizing it through a series of identical structures known as repeats.
Mutations in the gene encoding nebulin could generate an abnormal nebulin that results in nemaline myopathy—a condition of incurable neuromuscular disorder with several levels of severity ranging from muscle weakness, speech impediments, to respiratory problems.
Learning about the nebulin structures and how they actually communicate with actin can be essential to the new treatments’ development. In this research, the team was able to visualize myosin and actin straight in their native environment, the muscle, with the use of a high-efficiency microscopy technique known as electron cryo-tomography (cryo-ET).
Experiments were performed on mouse muscles that are identical to that of humans’, using methods optimized in labs at King’s College London’s Randall Centre for Cell & Molecular Biophysics. Amazingly, the clear absence of nebulin was demonstrated by heart muscle actin filaments.
The results show that every nebulin repeat gets bound with an actin subunit, revealing nebulin’s role as a ruler that gradates the actin filaments’ length. Each nebulin repeat communicates with every adjacent actin subunit, implying its role as a stabilizer. Lastly, the team proposes that nebulin can control the binding of actin and myosin, and thereby muscle contraction, by communicating with another protein known as troponin.
Motivated by the latest success, the team will now focus on exploring the details of myosin regulation structure—the motor filament of muscle. Such results can ultimately aid in understanding the complex details behind the heart and skeletal muscle contraction.
Source:
Journal reference:
Wang, Z., et al. (2022) Structures from intact myofibrils reveal mechanism of thin filament regulation through nebulin. Science. doi.org/10.1126/science.abn1934.