“Undruggable” proteins, whose structures and roles in disease are known but who appear unable to be targeted by drugs that will bind to them, present some of the hardest problems in disease treatment. According to new research from KAUST, many “undruggable” proteins can actually reveal drug-binding sites due to their molecular motion.
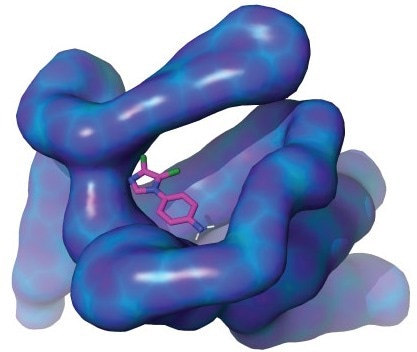 A KAUST-led team of researchers has revealed properties of the MIZ1 protein (illustrated above). Their findings could advance drug discovery for previously incurable diseases, including some forms of cancer. Image Credit: KAUST
A KAUST-led team of researchers has revealed properties of the MIZ1 protein (illustrated above). Their findings could advance drug discovery for previously incurable diseases, including some forms of cancer. Image Credit: KAUST
The BTB domain, a specific molecular area known to be essential to more than 350 proteins, is the subject of the study. It enables proteins to interact with one another and have an impact on intricate genetic and molecular signaling processes that are essential to the functioning of many cells.
As transcription factors, which regulate the activities of genes, more than 80 known BTB-containing proteins play a role in cancer. These cancers frequently result in death because the BTB domain has proven challenging to treat with medication.
Together with colleagues from the University of Michigan in the United States, the KAUST team performed a thorough analysis of the BTB domains’ molecular movements in three cancer-related proteins.
The findings revealed how molecular motion affects how well small molecules, also known as ligands, can bind to the BTB domain. This exposed cryptic binding sites, which are dynamic BTB domain regions that, in contrast to static structures, seem available to bind to ligands.
This means that some seemingly undruggable target proteins can now be reconsidered, with the firm hope of identifying novel lead compounds for anticancer drug development. The hero of our study, called the MIZ1 protein, is linked to c-MYC, the oncogene cancer-causing gene of over 70 percent of cancers, and can now be targeted for drug discovery campaigns.”
Łukasz Jaremko, Assistant Professor, Bioscience, Biological and Environmental Science and Engineering Division, King Abdullah University of Science and Technology
Even though it seems logical now, the researchers were surprised to learn how crucial protein movement could be in regulating ligand binding sites.
The next challenge is to fully comprehend the mechanisms that help molecular movements to make cryptic binding sites so difficult to detect and interact with, according to the study’s first author Vladlena Kharchenko, a former KAUST PhD student who is now a postdoctoral fellow at Albert Einstein College of Medicine in the US.
We also want to find these sites in other proteins, to advance the drug discovery process for many other currently undruggable proteins and ultimately give new hope for treating currently incurable diseases, including many forms of cancer.”
Vladlena Kharchenko, Study First Author and Postdoctoral Fellow, Albert Einstein College of Medicine
Source:
Journal reference:
Kharchenko, V., et al. (2022). Increased slow dynamics defines ligandability of BTB domains. Nature Communications. doi.org/10.1038/s41467-022-34599-6