Across the globe, soybean is considered to be an important food, oil, and feed crop worldwide.
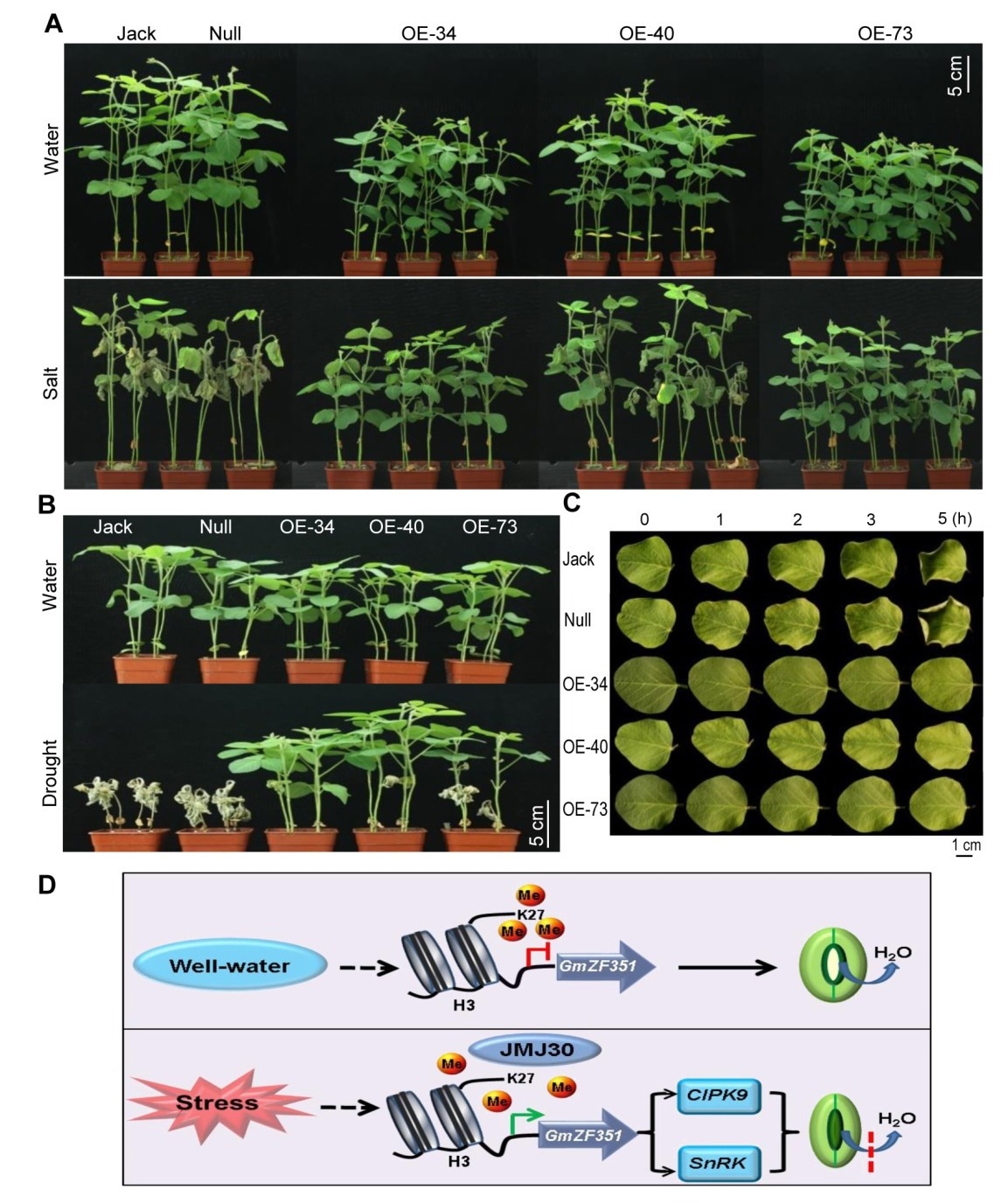 Performance of the GmZF351 transgenic soybean plants and its working model in stress tolerance. A. Performance of the GmZF351 transgenic plants under salt stress. Jack is the original control cultivar. Null is segregated from heterozygous transgenic plants and without transgene insertion. OE-34/40/73 indicate independent homozygous transgenic lines; B. Performance of the transgenic plants under drought stress; C; Comparison of water loss in detached leaves at different times; D. A working model of GmZF351 in stress response. Under normal well-watered condition, the histones H3K27 is highly methylated at the promoter region of GmZF351, blocking gene expression. Under stress, the histone demethylase JMJ30 is induced and the methylation of H3K27 is reduced, allowing activation of GmZF351 and subsequent downstream gene expression for stomata closure and stress tolerance. (Image by IGDB)
Performance of the GmZF351 transgenic soybean plants and its working model in stress tolerance. A. Performance of the GmZF351 transgenic plants under salt stress. Jack is the original control cultivar. Null is segregated from heterozygous transgenic plants and without transgene insertion. OE-34/40/73 indicate independent homozygous transgenic lines; B. Performance of the transgenic plants under drought stress; C; Comparison of water loss in detached leaves at different times; D. A working model of GmZF351 in stress response. Under normal well-watered condition, the histones H3K27 is highly methylated at the promoter region of GmZF351, blocking gene expression. Under stress, the histone demethylase JMJ30 is induced and the methylation of H3K27 is reduced, allowing activation of GmZF351 and subsequent downstream gene expression for stomata closure and stress tolerance. (Image by IGDB)
But in China, there is only a very limited production of soybean. For the yield of soybean to be increased, there is a pressing need to develop soybean varieties that could have the potential to grow and adapt to the immense areas of alkaline, saline, and arid lands.
A research group headed by Professor Jinsong Zhang from the Institute of Genetics and Developmental Biology (IGDB) of the Chinese Academy of Sciences (CAS) reported the mechanisms and roles of a transcriptional regulator GmZF351 in salt and drought stress tolerance in stable transgenic soybean plants.
This study was reported online in Journal of Integrative Plant Biology.
Earlier, Zhang’s group found out the fact that the gene is highly expressed in soybean seeds and facilitates oil accumulation in seeds that are in the developing stage. This gene displays either little or no expression at the seedling and/or vegetative stage. But when being subjected to salt and other stresses, the gene has been triggered for stress tolerance in soybean.
They discovered that histone demethylation might be included in such a transition. Under normal conditions, the histones have been highly methylated at the GmZF351 gene’s promoter region
On exposure to salt treatment, two histone demethylase genes, named JMJ30-1 and JMJ30-2, are triggered, leading to the dismissal of the histone H3K27me3 at the GmZF351 promoter and thus triggering the gene.
Furthermore, the researchers examined the cis-elements bound by the zinc finger protein called GmZF351. The element comprises two the motif CT(G/C) (T/A)AA for recognition. In stress tolerance and oil biosynthesis, all the downstream genes included have such features in their promoter regions. But the GmZF351 triggered various sets of genes for lipid accumulation in soybean seeds and in reaction to salt and drought stress.
As far as plants are concerned, it is expensive and energy-intensive to withstand stress and survive for future generations. Hence, stress tolerance and yield or storage accumulation should be completely considered at the time of plant growth and development to finish the life cycle.
The newly determine gene GmZF351 has the potential to promote both stress tolerance in seedlings and oil accumulation in seeds. This helps in balancing these two significant biological processes in various organs and at various developmental stages.
Comprehending the dual functions of GmZF351 might help gain better insights into the balancing mechanism of a crop for seed yield or storage and survival during crop domestication. Also, offering an inspiring basis and knowledge for developing new soybean varieties ideal for unfavorable saline lands and surroundings.
This study was financially supported by the National Natural Science Foundation of China and the Key Project of CAS.
Source:
Journal reference:
Li, X., et al. (2023) Vacancy accumulation mechanism at iron grain boundaries: The influence of grain boundary character and its coupling with grain size. Journal of Nuclear Materials. doi.org/10.1016/j.jnucmat.2023.154386.