Plants increasingly need to adapt quickly to preserve their existence as global warming continues to reshape ecosystems. Epigenetic memory, especially DNA methylation, is a key factor promoting such quick adaptability. A type of epigenetic alteration known as DNA methylation includes the insertion of a methyl group to the DNA’s cytosine bases, changing the DNA’s accessibility in chromatin and controlling gene expression. Environmental variables like a rise in temperature can cause changes in DNA methylation in the context of a warming climate.
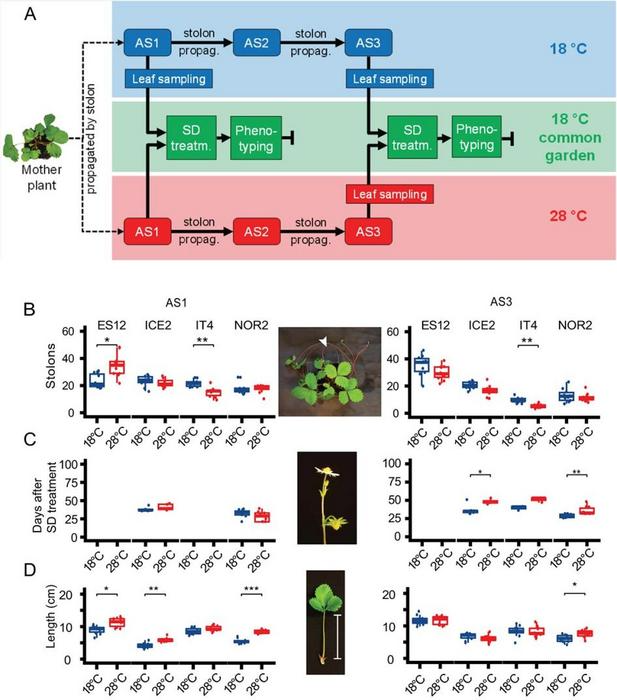 Experimental setup and phenotypic responses under common garden-conditions in Fragaria vesca plants after propagation at different temperatures for up to three asexual generations. Image Credit: Horticulture Research
Experimental setup and phenotypic responses under common garden-conditions in Fragaria vesca plants after propagation at different temperatures for up to three asexual generations. Image Credit: Horticulture Research
These epigenetic modifications are crucial in enabling plants to coordinate their development with changing environmental stimuli. A thorough knowledge of how these DNA methylation alterations impact plant phenotypes, particularly in response to warming temperatures, is still lacking.
A study titled “Warmer temperature during asexual reproduction induce methylome, transcriptomic, and lasting phenotypic changes in Fragaria vesca ecotypes” was published in Horticulture Research in July 2023.
Four European F. vesca ecotypes (ES12, ICE2, NOR2, IT4) were subjected to an experiment over three asexual generations in which they were exposed to temperatures of 18 °C and 28 °C to investigate the impact of temperature on phenotypic and epigenetic changes. The first asexual generation (AS1) at 28 °C saw enhanced stolon production for the ES12 ecotype, whereas the third (AS3) did not.

Image Credit: nnattalli/Shutterstock.com
On the other hand, in both AS1 and AS3, the IT4 ecotype showed reduced stolon production at 28 °C. According to AS3, the ICE2 and NOR2 ecotypes significantly delayed blooming at 28 °C, with statistical significance defined as 0.05 > p > 0.001. Plants from the ES12, ICE2, and NOR2 ecotypes exhibited petiole lengths that were larger when cultivated at 28 °C during AS1.
Only the NOR2 ecotype still had the longer petiole by AS3. The findings revealed a statistically significant difference between the growth temperature of the experiment and all phenotypic features examined, which can be maintained during asexual reproduction.
Bisulfite-sequencing of the genomic DNA samples from the ecotypes revealed observable changes in DNA methylation patterns between the two temperature settings, particularly in the CHG and CHH contexts, in further in-depth analyses at the molecular level. The most obvious change in methylation levels between the temperature ranges was seen in NOR2.
Methylation profiles of ecotypes growing at 18 °C and 28 °C showed significant differences, according to Principal Component Analysis (PCA). All ecotypes experienced significant alterations in both hypo- and hypermethylation, with the CHH context showing the biggest temperature-specific methylation increases.
Notably, transcription start sites (TSS) and transcription termination sites (TTS) were shown to be associated with methylation alterations. Hypermethylation is usually present in areas where CHG and CHH methylation are different. Approximately 3,500 to 5,000 differentially expressed genes (DEGs) in various ecotypes were found to have transcriptome modifications connected to temperature increase at the same time.
Additionally, this study investigated the methylation and expression patterns that are ecotype-specific for genes involved in the metabolism of gibberellin, flowering time, and epigenetic processes. The absolute multiple change of 1,318 associated differentially expressed and differentially methylated genes (DEDMGs) was found to be >1.5 across ecotypes with three or fewer.
In conclusion, the study supports the presence of a temperature-related epigenetic memory effect in F. vesca ecotypes by showing that temperature fluctuations during asexual propagation cause significant hereditary epigenetic and phenotypic alterations.
This ground-breaking research advances the current understanding of plant adaptability and paves the way for using epigenetic memory to create crops that are more climate-smart.
Source:
Journal reference:
Zhang, Y., et al. (2023). Warmer temperature during asexual reproduction induce methylome, transcriptomic, and lasting phenotypic changes in Fragaria vesca ecotypes. Horticulture Research. doi.org/10.1093/hr/uhad156