Reviewed by Danielle Ellis, B.Sc.Dec 4 2023
Upon infection or immunization, antibodies produced by all jawed vertebrates serve to bind and neutralize pathogens.
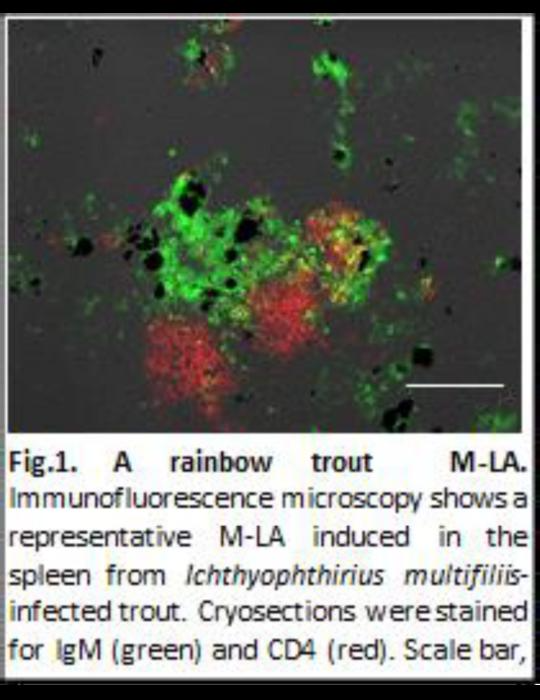 Immunofluorescence microscopy shows a representative M-LA induced in the spleen from Ichthyophthirius multifiliis-infected trout. Cryosections were stained for IgM (green) and CD4 (red). Scale bar, 100 μm. Groups of dark cells are MMCs. Image Credit: Yasuhiro Shibasaki
Immunofluorescence microscopy shows a representative M-LA induced in the spleen from Ichthyophthirius multifiliis-infected trout. Cryosections were stained for IgM (green) and CD4 (red). Scale bar, 100 μm. Groups of dark cells are MMCs. Image Credit: Yasuhiro Shibasaki
In warm-blooded species like mammals, robust and enduring antibody responses are generated in secondary lymphoid microstructures (SLMs), with germinal centers (GCs) serving as the focal point.
Despite the absence of GCs or analogous SLMs in cold-blooded vertebrates, such as fish, these species exhibit significant and persistent antibody responses for several months. The question of how and where antibody responses occur in species lacking GCs or similar SLM structures has intrigued scientists for decades.
A recent study, featured on the cover of Science Immunology, challenges the conventional understanding of immune responses in cold-blooded species.
Researchers at the University of Pennsylvania’s School of Veterinary Medicine (Penn Vet) have uncovered that contrary to previous beliefs, bony fish induce antibody responses in primordially organized SLMs, which function similarly to GCs in warm-blooded animals.
Specifically, the study identifies the formation of large aggregates of highly proliferating B cells (antibody-producing cells) and T cells (cells aiding B cells in antibody production) in the spleen of fish upon infection or immunization. These newly induced B and T cell zones are located in close proximity to melanomacrophage centers (MMCs), tissue areas containing dark-colored melanomacrophages that retain antigens during infection.
Image Credit: ustas7777777/Shutterstock.com
These recently discovered MMC-associated lymphoid aggregates (M-LAs) contain numerous antigen-specific B cells, underscoring their crucial role in the immune response. Furthermore, similar to processes observed in GCs, B cell clonal expansion and somatic hypermutation occur within M-LAs.
Our findings challenge the former dogma that fish do not contain specific lymphoid microenvironments where immune responses are generated while revealing a previously unknown type of SLM in jawed vertebrates.”
J. Oriol Sunyer, Study Corresponding Author and Professor, Immunology, School of Veterinary Medicine, University of Pennsylvania
Sunyer added, “This discovery has far-reaching implications for our understanding of the immune system’s evolution and its potential applications in various fields, from fish vaccinology to human medicine.”
The study introduces a fresh outlook on the initiation of immune responses in vertebrates, providing novel insights into comprehending the fundamentally conserved principles governing the functionality of M-LAs and GCs.
For instance, fish M-LAs are highly polyclonal structures, thus resembling newly identified mammalian GCs that operate in polyclonal settings. Therefore, the study of fish M-LAs is likely to shed light on the mechanisms by which both polyclonal GCs and M-LAs are formed.”
J. Oriol Sunyer, Study Corresponding Author and Professor, Immunology, School of Veterinary Medicine, University of Pennsylvania
From a practical standpoint, these discoveries hold significant importance for developing more efficacious knowledge-driven vaccines for fish. Disease and health management pose major challenges for the burgeoning aquaculture industry, both in the United States and globally.
Although fish vaccines have played a crucial role in nearly eliminating various fish diseases, the effectiveness of many vaccines against both longstanding and emerging fish pathogens is hampered by our limited understanding of how immune responses are triggered in these species.
Now that we know where and how antibody responses are induced in fish, the study of M-LAs will identify correlates of immune activation and protection that will pave the road for the screening and development of more efficacious and safer vaccines and adjuvants for the aquaculture industry.”
J. Oriol Sunyer, Study Corresponding Author and Professor, Immunology, School of Veterinary Medicine, University of Pennsylvania
Source:
Journal reference:
Shibasaki, Y., et al. (2023) Cold-blooded vertebrates evolved organized germinal center–like structures. Science Immunology. doi.org/10.1126/sciimmunol.adf1627.