Reviewed by Danielle Ellis, B.Sc.Jan 29 2024
For terrestrial creatures, including humans, to survive, they must drink plenty of water and eat enough salt. Neural pathways comprising many brain regions intriguingly control both thirst and salt appetite.
 This study reveals key aspects of the feedback mechanisms regulating the thirst and salt appetite. Image Credit: Tokyo Institute of Technology
This study reveals key aspects of the feedback mechanisms regulating the thirst and salt appetite. Image Credit: Tokyo Institute of Technology
Previous research revealed that consuming water or salt reduces thirst and salt appetite before the digestive tract has a chance to absorb the substances. This suggests that the digestive organs have sensing and feedback mechanisms that aid in the real-time modulation of thirst and salt appetite in response to food and drink. Unfortunately, the specifics of these fundamental systems have remained obscure despite a great deal of research on the subject.
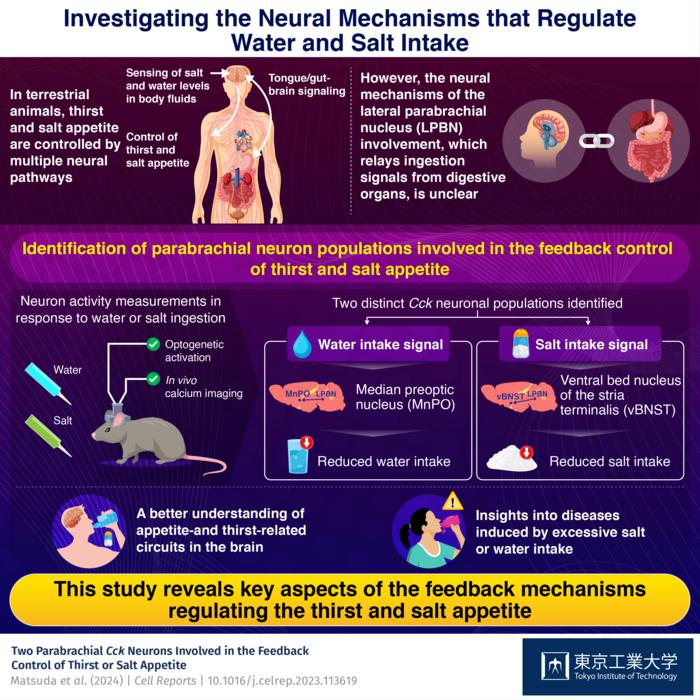
In an effort to clarify this issue, a Japanese research team recently carried out a thorough investigation into the parabrachial nucleus (PBN), which serves as the brain’s relay hub for ingestion impulses originating from the digestive system.
On January 23, 2024, Cell Reports published their most recent publication, which was co-authored by Assistant Professor Takashi Matsuda of the Tokyo Institute of Technology.
Genetically modified mice were used by the researchers in several in vivo studies. Using optogenetic (and chemogenetic) alterations and in vivo calcium imaging tools, they were able to see and manipulate, with light and chemicals, the activation or inhibition of certain neurons in the lateral PBN (LPBN).

Image Credit: Alexeysun/Shutterstock.com
The mice were given water or salt water during the trials, either in normal or water- or salt-depleted situations, and the researchers observed the accompanying drinking habits and brain activity.
By doing this, the researchers were able to distinguish between two different subgroups of cholecystokinin mRNA-positive neurons in the LPBN that were activated in response to salt and water ingestion. While the neuronal population responding to salt intake projects to the stria terminalis’s ventral bed nucleus (vBNST), the neuronal population responding to water intake projects from the LPBN to the median preoptic nucleus (MnPO).
Interestingly, even in mice that had previously been denied either salt or water, the researchers found that if they artificially stimulated these neuronal populations through optogenetic (genetic control using light) trials, the mice drank far less water and consumed less salt. In a similar vein, the mice drank more water and salt than usual when the researchers artificially blocked these neurons.
Consequently, these LPBN neuronal populations participate in feedback mechanisms that decrease hunger and thirst when consuming water or salt, potentially assisting in the prevention of excessive salt or water consumption.
Together with their earlier neurological research, these findings demonstrate that MnPO and vBNST, which integrate promotion and suppression signals from multiple brain regions, are the control centers for salt appetite and thirst.
Understanding brain mechanisms controlling water and salt intake behaviors is not only a significant discovery in the fields of neuroscience and physiology, but also contributes valuable insights to understand the mechanisms underlying diseases induced by excessive water and salt intake, such as water intoxication, polydipsia, and salt-sensitive hypertension.”
Takashi Matsuda, Assistant Professor, Tokyo Institute of Technology
Masaharu Noda, Professor, added, “Many neural mechanisms governing fluid homeostasis remain undiscovered. We still need to unravel how the signals for inducing and suppressing water and salt intake, accumulated in the MnPO and vBNST, are integrated and function to control intake behaviors.”
Source:
Journal reference:
Matsuda. T., et.al., (2024). Two parabrachial Cck neurons involved in the feedback control of thirst or salt appetite. Cell Reports. doi.org/10.1016/j.celrep.2023.113619