Researchers from Stanford University and LMU have uncovered how cells renew their endoplasmic reticulum’s protein factories.
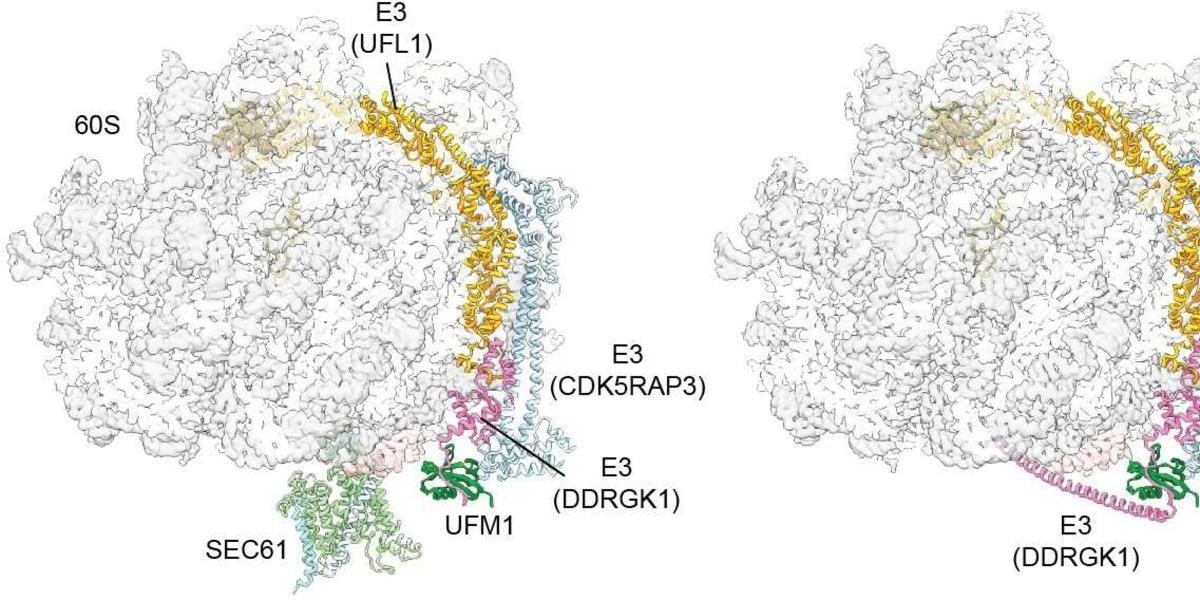 The protein UFM1 acts as a molecular wedge, facilitating the cleavage of the Sec channel. Image Credit: T. Becker, Nature 2024.
The protein UFM1 acts as a molecular wedge, facilitating the cleavage of the Sec channel. Image Credit: T. Becker, Nature 2024.
One of the essential functions of life is the cell’s synthesis of proteins. This is how the amino acid sequence of proteins is translated from the genetic code of the genome. The procedure is intricate and has been thoroughly researched for many years.
Specialized molecular machines called ribosomes, which comprise a large and a small subunit, are responsible for protein biosynthesis. These protein factories must be disassembled (recycled) at the conclusion of protein biosynthesis in order to be prepared for the subsequent translation cycle.
The recycling of ribosomes at the so-called endoplasmic reticulum (ER) has now been demonstrated by a team led by Professor Roland Beckmann, Dr Thomas Becker, and Ivan Penchev from LMU’s Gene Center Munich, in cooperation with researchers at Stanford University led by Professor Ron Kopito.
During this process, they found that a unique E3 ligase plays a crucial role in recycling by joining a small protein modification known as UFM1 to the large ribosomal subunit. The esteemed journal Nature has published their findings.
Detailed Insights into the Recycling of Ribosomes
Normally, ribosomes are observed to be floating in the cytoplasm. According to Becker, “We know exactly how the recycling works here.” On occasion, though, they can be found at the endoplasmic reticulum, a continuous membrane network that spans the entire cell.
Even though the cytosol is where many proteins start, they eventually need to be transported to other organelles like mitochondria and chloroplasts, among many others. The entire translation machinery docks to the ER membrane if a protein is synthesized there. A protein-conducting channel (SEC61), which can move proteins across the membrane or insert them into the membrane during synthesis, helps to achieve this.
Following translation completion, an additional recycling step - particular to the ER membrane-occurs. Specifically, the ribosome’s large subunit must reattach itself to the protein-conducting channel. Beckmann's team has successfully illustrated this substep's mechanism: upon translation completion, the E3 ligase identifies the large subunit of the ribosomes.
It places – figuratively speaking – a small wedge, the protein UFM1, at the large subunit. This produces a stable complex out of the modified 60S subunit and the E3 ligase. Simultaneously, it causes the large subunit to detach from SEC61. This is a very important step to ensure that the large subunit is returned to the cytosol and available for the next round.”
Dr Thomas Becker, Gene Center Munich, Ludwig-Maximilians-Universität München
Source:
Journal reference:
Paul A. DaRosa, A. P., et al., (2024) UFM1 E3 ligase promotes recycling of 60S ribosomal subunits from the ER. Nature. doi.org/10.1038/s41586-024-07073-0