 Image Credit: Center for Molecular Medicine of the Austrian Academy of Sciences
Image Credit: Center for Molecular Medicine of the Austrian Academy of Sciences
With the help of AI-assisted image recognition, this automated high-throughput method opens up completely new applications in a variety of fields, from basic cell biology to drug discovery. The research has been published in the journal Nature Cell Biology.
Life would not be possible without proteins. They give cells their structural foundation, function as metabolic regulators or transporters, or function as signaling molecules to let cells interact with their surroundings. Each of these roles can be completed only when the proteins are positioned correctly within the cell.
Since a protein's location frequently affects its properties as well, controlling a protein's localization within a cell also controls its function.
It is crucial to precisely identify and monitor the location of proteins within the cell to comprehend and investigate their function. Proteins frequently move quickly between various organelles and cell compartments. They are frequently connected to a fluorescent, brilliantly shining protein component to be seen under a microscope.
Technical challenges have been encountered with this approach, though: normally, the fluorescent component could only be attached to one protein at a time, and labeling multiple proteins required fixing and killing of the cells.
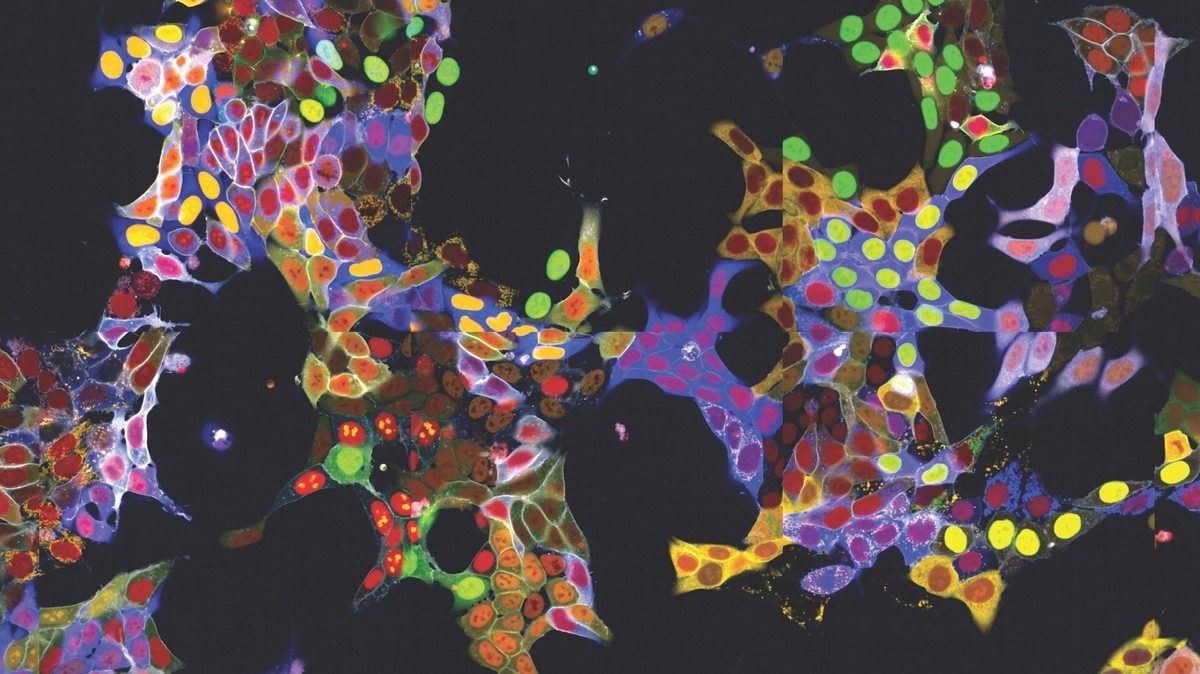
“Visual proteomics Cells” (abbreviated vpCells), a novel technique described by Stefan Kubicek's group, enables proteins to be fluorescently labeled while maintaining their native regulatory mechanisms. Using a technique known as the “multiplex approach,” vpCells can fuse multiple proteins with a fluorescent tag at once rather than labeling one at a time.
In 2020, Kubicek's group published a description of an earlier version of this technique for the investigation of metabolic enzymes. It has now been enhanced and broadened in three directions:
Firstly, by genetically attaching fluorescent proteins to the proteins under study using the CRISPR/Cas9 gene-editing tool, vpCells can label every protein that could exist. For this purpose, Kubicek's group has constructed a genome-wide “library” that allows for the systematic functional exploration and fluorescent marking of every human protein that could exist.
Secondly, vpCells employ five complementary colors in total rather than just one fluorescent color. Two distinct proteins that need to be monitored are labeled in every cell. Furthermore, a different color marking is applied to help identify individual clones more clearly. To further help distinguish individual cells, two additional colors are used to indicate the cell membrane and nucleus.
Thirdly, this color scheme makes it possible to distinguish and optically identify the various proteins in addition to producing aesthetically pleasing images. Usually, after imaging, this necessitates intricate DNA sequencing to identify the tagged protein.
On the other hand, using only fluorescence microscopy images, the vpCells approach makes it possible to train an AI-assisted image recognition system to identify which protein is marked in which cell.
Two applications have already shown the method's usefulness: On the one hand, over 4,500 cell lines representing over 1,100 proteins were created. These cell lines were used to describe the localization of the proteins in their basal state and to train the AI models.
However, Kubicek's team employed the living reporter cells to investigate a particular research question: they looked at how over a thousand small molecules affected 61 proteins that are important to cancer cells.
According to the researchers, 44 of the tested substances changed the quantity or location of specific proteins within six hours. One of the chemicals was found to be an inhibitor of protein transport from the cell nucleus, which functions similarly to a medication currently licensed for treating multiple myeloma, a blood-forming system cancer.
These results provide a first glimpse into the versatility of the vpCells method, we expect many more future applications, from fundamental cell biology to applied drug discovery.”
Stefan Kubicek, Center for Molecular Medicine of the Austrian Academy of Sciences
Source:
Journal references:
Reicher, A., et al. (2024) Pooled multicolor tagging for visualizing subcellular protein dynamics. Nature Cell Biology. doi.org/10.1038/s41556-024-01407-w