Reviewed by Lauren HardakerMay 23 2025
Most people associate cholera with contaminated water and severe outbreaks in vulnerable regions. But behind the scenes, cholera bacteria are locked in microscopic conflict - battles that may influence how pandemics develop.
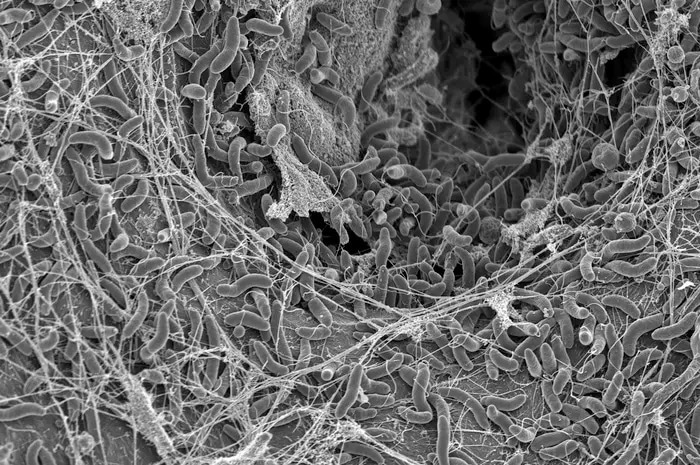 Vibrio cholerae bacteria, photo taken with a Scanning Electron Microscope. Image Credit: Graham Knott & Melanie Blokesch (EPFL)
Vibrio cholerae bacteria, photo taken with a Scanning Electron Microscope. Image Credit: Graham Knott & Melanie Blokesch (EPFL)
In addition to fighting against antibiotics and public health initiatives, bacteriophages (phages), which are viruses that infect and kill bacteria, are a constant threat to cholera bacteria.
These viruses have the power to create or destroy entire epidemics, not just individual infections. In fact, by eliminating the bacterium that causes cholera, Vibrio cholerae, some bacteriophages are believed to reduce the scope and length of cholera outbreaks.
Since the 1960s, the ongoing seventh cholera pandemic has been driven by the “seventh pandemic El Tor” (7PET) strains of V. cholerae, which have spread globally in successive waves. In response to persistent phage threats, these bacteria have evolved a variety of defense mechanisms.
For instance, numerous bacterial strains possess mobile genetic elements that equip them with anti-viral capabilities. This raises the question: why are certain cholera strains particularly adept at evading phage attacks? Could this ability either facilitate or amplify the pathogen’s devastating impact on human populations?
One outbreak of note occurred in the early 1990s, when cholera swept through Peru and much of Latin America, infecting over a million people and causing thousands of deaths. The responsible V. cholerae strains belonged to the West African South American (WASA) lineage. It is still unclear why these WASA strains led to such a widespread outbreak in Latin America.
Now, researchers at EPFL’s Global Health Institute, led by Melanie Blokesch, have uncovered part of the answer. A study published in Nature Microbiology shows that the WASA lineage acquired several unique bacterial immune systems that protected it against a range of phages. This phage resistance may have contributed to the outbreak’s extensive spread.
The researchers examined Peruvian cholera strains from the 1990s, assessing their resistance to key phages, particularly ICP1, a prevalent virus that has been thoroughly studied in the cholera-endemic region of Bangladesh, where it is believed to help limit cholera outbreaks. Surprisingly, the Peruvian strains exhibited immunity to ICP1, unlike other strains representative of the 7th pandemic.
By removing specific segments of the cholera strain’s DNA and transferring these genes into other bacterial strains to evaluate their function, the team identified two significant defense regions within the WASA strain’s genome: the WASA-1 prophage and the genomic island known as Vibrio seventh pandemic island II (VSP-II).
These genomic areas encode specialized anti-phage systems that collaborate to form a bacterial immune system capable of defending against phage infections.
One of these systems, WonAB, initiates an “abortive infection” response that eliminates infected cells before phages can replicate, sacrificing a few bacteria to protect the larger population.
This approach differs from traditional bacterial immune systems, such as restriction-modification systems, which degrade phage DNA upon entry into the cells.
Instead, it stops the phage from replicating but only after it has already hijacked the cholera bacterium's cellular machinery, effectively locking the infected bacteria in a standoff but at least the phage does not spread.
David Adams, Study Lead Author, Ecole Polytechnique Fédérale de Lausanne
Two additional systems, GrwAB and VcSduA, provide distinct protective roles: GrwAB targets phages with chemically modified DNA, a tactic used by phages to disguise their genomes and evade other bacterial immune defenses.
Conversely, VcSduA acts against various families of viruses, including another common “vibriophage,” offering layered protection that broadens the bacterial population’s resistance capabilities.
In essence, the WASA lineage of V. cholerae is protected from its primary phage predator, ICP1, and carries a broader set of anti-phage defense systems that allow it to resist multiple types of bacteriophages.
With growing interest in phage therapy as a potential alternative to antibiotics, understanding how epidemic bacteria evade phage attack is increasingly important.
If bacteria like V. cholerae can acquire viral defense systems and enhance their ability to spread, this could reshape how cholera is monitored, treated, and controlled. The findings underscore the need to consider phage-bacteria interactions as a key factor in infectious disease research and outbreak management.
Source:
Journal reference:
Adams, D. W., et al. (2025) West African–South American pandemic Vibrio cholerae encodes multiple distinct phage defence systems. Nature Microbiology. doi.org/10.1038/s41564-025-02004-9.