Reviewed by Lexie CornerJun 3 2025
Researchers from the Max Planck Institute of Psychiatry (MPI), Helmholtz Munich, and the University of Sydney have identified biological mechanisms shared across different mental illnesses. They conducted the study using postmortem brain tissue samples taken from the dorsolateral prefrontal cortex (DLPFC).
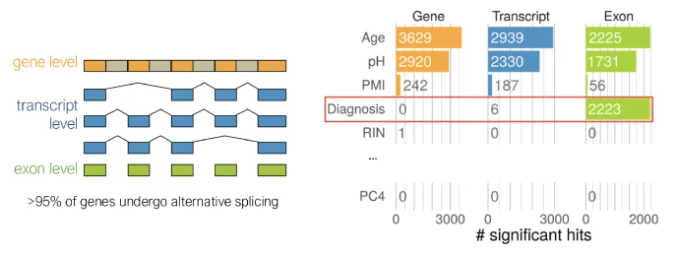 The DNA (gene level) is transcribed into mRNA (transcript level). The mRNA consists of introns and exons. Introns are removed, while exons are spliced, recombined, and then translated into proteins. Image Credit: Max Planck Institute of Psychiatry
The DNA (gene level) is transcribed into mRNA (transcript level). The mRNA consists of introns and exons. Introns are removed, while exons are spliced, recombined, and then translated into proteins. Image Credit: Max Planck Institute of Psychiatry
The DLPFC, often associated with logical reasoning and emotional regulation, is frequently linked to mental health conditions. The study analyzed samples from both healthy individuals and those diagnosed with mental illnesses, most commonly schizophrenia.
A distinguishing aspect of this research is the integration of multiple layers of genetic data.
In contrast to studies that look at gene expression as a whole, we analyzed the exon level to better understand the structure of the genes. This detailed approach gave us a better understanding of how genetic variation influences disease risk.
Karolina Worf, Study First Author, Helmholtz Munich
Exons are the regions of a gene that contain key information. They provide the instructions for building proteins and also determine which protein variants are produced through a process called alternative splicing. This process occurs in more than 95 % of human genes.
Including exon-level data in the analysis was a crucial step. Differences between psychiatric patients and healthy controls were significant at the exon level but not at the overall gene level.
The risk of developing a psychiatric disorder seems to, therefore, not just depend on what genes you have, but how your genes are expressed.
Janine Knauer-Arloth, Leader, Project Group Medical Genomics, Max Planck Institute of Psychiatry
The researchers incorporated multiple types of genetic data, including single-nucleotide polymorphisms (SNPs), rare genetic variants, and polygenic risk scores. These scores estimate an individual’s disease risk by combining the effects of many relevant genetic variants. This approach enabled the identification of disruptions in pathways related to circadian rhythm, cortisol regulation, and dopamine signaling across all three psychiatric conditions studied.
The results suggest that psychiatric disorders share underlying biological mechanisms. Over time, this information may help researchers move toward classifying these conditions not only by symptoms but also by biological factors. This shift could support the development of more accurate diagnoses and targeted treatments.
Source:
Journal reference:
Worf, K., et al. (2025) Exon-variant interplay and multi-modal evidence identify endocrine dysregulation in severe psychiatric disorders impacting excitatory neurons. Translational Psychiatry. doi.org/10.1038/s41398-025-03366-8.