Reviewed by Lexie CornerJun 6 2025
A team of researchers used Automated Meiotic Mapping (AMM), a method developed at UT Southwestern Medical Center, to identify a gene that may play a key role in regulating food intake.
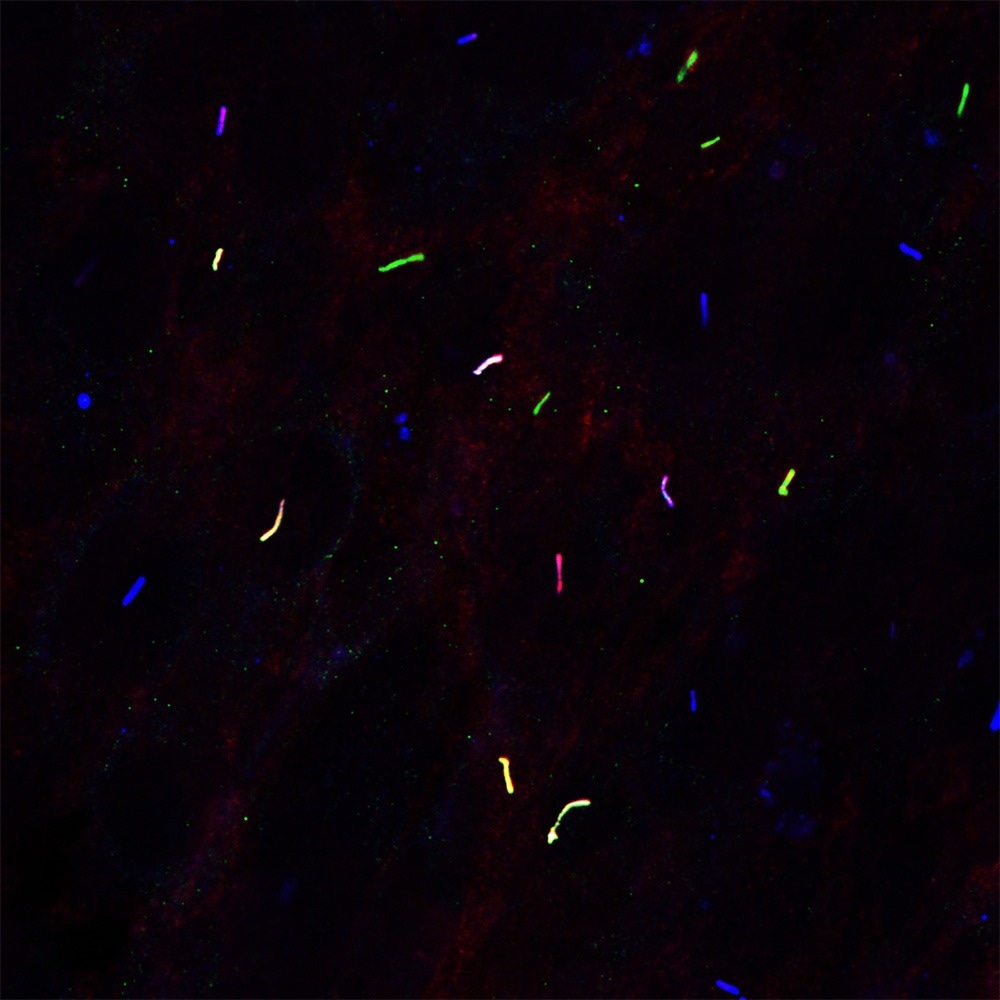 This fluorescent microscopy image shows GPR45 (green) localized in the primary cilia (blue), where it facilitates the transport of Gαs (red) into the cilia of hypothalamic cells. Image Credit: UT Southwestern Medical Center
This fluorescent microscopy image shows GPR45 (green) localized in the primary cilia (blue), where it facilitates the transport of Gαs (red) into the cilia of hypothalamic cells. Image Credit: UT Southwestern Medical Center
The findings, published in Science, could help inform new approaches to addressing obesity, which affects more than 40 % of adults in the United States and over a billion people worldwide.
This research uncovers a previously unknown signaling pathway in tiny, antenna-like structures on brain neurons that plays a critical role in controlling appetite, opening new doors for anti-obesity treatments.
Zhao Zhang, Ph.D., Study Leader and Assistant Professor, Center for the Genetics of Host Defense and Internal Medicine, UT Southwestern
The introduction of weight-loss medications in recent years has changed the health care field. These drugs not only support long-term weight management but also improve cardiovascular health, blood sugar levels, and blood pressure and cholesterol control.
This new study points to possible targets for appetite control that could be used on their own or alongside existing weight-loss treatments.
Body weight is thought to be influenced by both genetic and environmental factors. However, identifying specific gene variations linked to weight gain in humans is challenging due to differences in diet and lifestyle, according to Dr. Zhang. To address this, he and his colleagues used a forward genetics approach, supported by Automated Meiotic Mapping (AMM), to study the genetics of obesity.
UT Southwestern Nobel Laureate and study co-author Dr. Bruce Beutler, Director of the Center for the Genetics of Host Defense and Professor of Immunology and Internal Medicine, applied the AMM method to mice. His team induced genetic mutations, screened for traits of interest, and used genotypic analysis along with high-speed computing to identify the related mutations in real time. This approach combines artificial intelligence with statistical analysis.
Using this method, the researchers studied two separate mutations in the gene Gpr45. Both mutations caused rats to gain excess weight, even on a regular diet. When Gpr45 was deleted in healthy mouse embryos using CRISPR gene-editing technology, the same effect occurred. This showed that the gene plays an important role in regulating body weight.
Further tests found that the animals’ weight gain, which started at around six weeks of age, was caused by significantly increased food intake compared to their littermates without the mutations.
Dr. Zhang and his team investigated whether GPR45 - the protein encoded by the Gpr45 gene - is present in the hypothalamus, a part of the brain known to control eating behavior. Previous studies at UTSW and elsewhere have shown that the hypothalamus plays a key role in appetite regulation. The researchers confirmed that GPR45 is active in hypothalamus neurons and is located in small cellular structures called primary cilia.
Primary cilia also contain proteins made by other appetite-related genes, such as MC4R. Mutations in MC4R, as well as certain rare genetic conditions called ciliopathies, have been linked to childhood obesity. However, the role of obesity-related proteins in primary cilia remains unclear.
Dr. Zhang’s team found that GPR45 helps move a protein called Gαs from inside the cell to the primary cilia. There, it activates MC4R to help regulate appetite. The mutations identified through forward genetics seem to block this process. Without GPR45 present in the cilia, MC4R is not activated, and the animals continue to overeat.
According to Dr. Zhang, there are currently two drugs that target MC4R. However, the U.S. Food and Drug Administration has only approved them to treat obesity caused by rare genetic disorders that affect the MC4R pathway.
Because this gene is also active in other tissues and the drugs might affect similar receptors elsewhere, they are not suitable for more common forms of obesity. Dr. Zhang suggested that developing drugs to enhance GPR45 activity could offer a new strategy for treating obesity.
Source:
Journal reference:
Xun, Y., et al. (2025) GPR45 modulates Gα s at primary cilia of the paraventricular hypothalamus to control food intake. Science. doi.org/10.1126/science.adp3989.