Reviewed by Lauren HardakerSep 17 2025
A study at the University of Hawaiʻi at Mānoa by researchers from the John A. Burns School of Medicine (JABSOM) found that losing a crucial male fertility gene causes infertility and alters the expression of hundreds of other genes.
 Image credit: Lightspring/Shutterstock.com
Image credit: Lightspring/Shutterstock.com
Professor Dr. Monika Ward of the Department of Anatomy, Biochemistry, and Physiology at the Yanagimachi Institute for Biogenesis Research (YIBR) conducted the study. The researchers have been studying a gene known as Zfy, which is encoded by the zinc finger Y. Located on the Y chromosome in both mice and humans, Zfy is recognized as a male fertility factor that influences male fertility. In mice, Zfy exists in two forms: Zfy1 and Zfy2.
The researchers initially employed CRISPR-Cas9 to create knockout mice that lacked Zfy1, Zfy2, and both Zfy1 and Zfy2 genes, dubbed Zfy DKO (Zfy double knockout). They discovered that Zfy DKO men were entirely infertile and had significantly defective sperm. In the most extreme cases, Zfy DKO males could not make any sperm. These findings were published in the journal Biology of Reproduction in 2022.
The researchers then used assisted reproduction technologies (ART) to create more infertile Zfy DKO males, allowing the study of these mice to continue. They employed intracytoplasmic sperm injection (ICSI) and round spermatid injection (ROSI), which were pioneered at the University of Hawaiʻi by YIBR founder Ryuzo Yanagimachi.
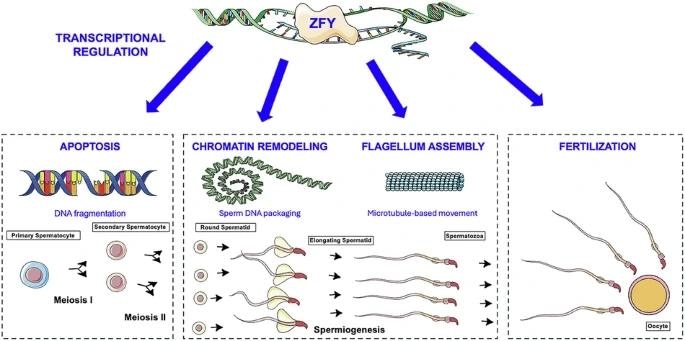
Graphical abstract
In a recent study published on August 27th in Cell Death and Differentiation, the team demonstrates the molecular repercussions of Zfy depletion.
The researchers demonstrated that without Zfy, hundreds of genes become deregulated, meaning they are expressed strongly or too weakly. These genes include those important for sperm production, DNA packing and organization, and cell death.
The researchers linked deregulation of these genes to alterations in the testes and sperm. They discovered that sperm precursor cells in the testes were dying prematurely, and that sperm, if formed, had DNA that was not correctly condensed and hence susceptible to harm.
This work really pushes forward our understanding of how this important Zfy gene works. We identified pathways and other genes that are affected and we can now study how exactly Zfy regulates them.
Dr. Monika Ward, Professor, Department of Anatomy, Biochemistry & Physiology, University of Hawaiʻi
Dr. Ward added, “This study exemplifies a critical role undergraduate and graduate students play in research at the University of Hawaiʻi. The first author of the paper, and a person who performed most of the work, is a recently graduated PhD student in the Developmental and Reproductive Biology (DRB) graduate program, Hayden Holmlund. Hayden now continues his academic career as a post-doctoral fellow in California. Some of the experiments were performed by an undergraduate INBRE student, Benazir Yarbabaeva, who has just started as a MS student in DRB program to continue her explorations of Zfy DKO sperm.”
“Finally, the study exemplifies the core mission of the YIBR,” Dr. Ward further added.
The YIBR promotes cooperation in developmental biology, reproductive biology, and other fields.
Dr. Ward added, “The new study was performed with contributions from colleagues from France and England.”
The study paves the way for further investigations into the regulatory role of Zfy in sperm production and marks a substantial advancement in our knowledge of male fertility regulation. The basic knowledge gained from basic research using mice as a model has translational implications and targets a particular health need: managing male fertility and treating male infertility.
The study shows how the absence of a crucial reproductive gene encoded on the Y chromosome may affect the expression of other genes. The researchers discovered that mice deficient in Zfy genes are sterile.
When they examined the effects of Zfy deletion on the transcriptome of sperm precursor testicular cells, spermatocytes, and spermatids, they discovered hundreds of dysregulated genes. These included those related to chromatin remodeling, apoptosis, sperm production, and sperm function.
Source:
Journal reference:
Holmlund, H. et al. (2025) T-STAR: Large-scale transcriptomic analyses reveal downstream target genes of ZFY1 and ZFY2 transcription factors in male germ cells. Cell Death & Differentiation. doi.org/10.1038/s41418-025-01569-6.