The discipline that correlates chemical changes with the movement of electrons is known as electrochemistry. Particularly, electrochemistry studies the generation of electricity by chemical processes and vice versa.
Electrochemistry. Image Credit: Visuta/Shutterstock.com
The fundamentals of electrochemistry are based on oxidation-reduction (or “redox”) reactions, which involve changes in the oxidation state of one or more elements. The father of electrochemistry is Alessandro Volta, who at the end of the 18th century discovered that electricity could be produced by placing silver and zinc discs on a brine-soaked cloth, creating the first-ever prototype of battery.
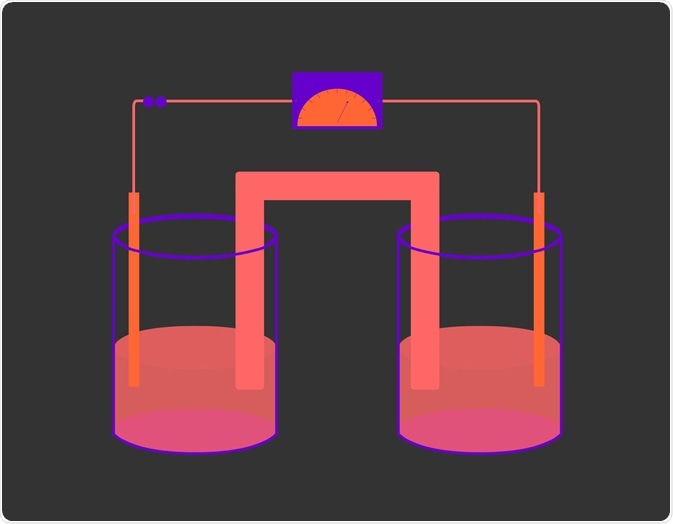
Voltaic cells (named after Volta) consist of two compartments called half-cells: the anode, where oxidation occurs, and the cathode, which is the half-cell where reduction takes place. For a redox reaction to occur, the two substances in each respective half-cell must be connected by a closed circuit so that electrons can flow from the reducing agent to the oxidizing agent. Moreover, a salt bridge is also required to maintain electrical neutrality and allow the reaction to continue.
The direction in which electrons move depends on the difference in potential energy between the anode and cathode. Electrons move from areas of higher potential energy to areas of lower potential energy. In voltaic cells the anode has higher potential energy, therefore electrons move from anode to cathode. The potential difference (Ecell) between the two electrodes is measured in volts (V).
There is a relationship between the cell potential and the thermodynamics of the electrochemical reactions. The variation in Gibbs free energy (ΔG), which indicates if a reaction occurs spontaneously, is related to Ecell via the equation: ΔG = -nFEcell. For voltaic cells, ΔG is always negative, therefore reactions occur spontaneously.
Electrolytic cells are very similar to voltaic cells. However, the reactions are not spontaneous and, to generate a potential difference between the electrodes and cause the electrons to flow, an external source of electrical energy is required.
Real-life applications – Tackling corrosion
One of the amazing aspects of electrochemistry is the fact that it is applied every day in our lives. From personal devices to industrial processes, electrochemical reactions are used for numerous applications.
A very common application used everywhere is galvanization, a process where a ferrous material (e.g., steel) is coated with a thin layer of zinc. The layer protects the surface of the metal part from atmospheric agents, preventing the formation of iron oxides and therefore stopping corrosion. Galvanization is a cost-effective solution for coating steel parts, specifically those significantly exposed to the environment over their lifetime.
Corrosion is also prevented by using “sacrificial anodes”. Sacrificial anodes are created from a metal alloy with a more negative electrochemical potential than the metal that needs to be protected. Consequently, corrosion will take place at the sacrificial anode rather than the metal it is protecting.
The revolution of portable devices
The main applications of electrochemistry are certainly found in batteries, which generally contain at least one voltaic cell and are used for the storage and generation of electricity.
When the electrochemical reactions are irreversible and the materials in the electrodes are exhausted, the batteries are non-rechargeable and disposable (primary batteries). These are the common batteries usually found in flashlights and remote controls.
On the other hand, the batteries used in cars and portable devices are defined as secondary batteries. In this case, the electrochemical reactions are reversible and the batteries can be recharged by using an external electrical source.
Over the last three decades, lithium-ion (Li-ion) batteries have revolutionized the world of technology, particularly concerning portable devices. Their advent has been a game-changer, as evidenced by the 2019 Nobel Prize in Chemistry.
Li-ion batteries can store much more energy per unit weight or volume compared to other rechargeable battery systems. Due to the use of non-aqueous electrolytes, Li-ion batteries can achieve high voltages (3-5 V per cell) and have a lower self-discharge.
Powering vehicles with electrochemical reactions
Another way of generating electricity from electrochemical reactions is with fuel cells. These devices have been studied for decades although so much research is still in progress. Fuel cells find applications in the automotive sector since they can produce enough energy to power buses, cars, and other vehicles.
The electrochemical reaction in fuel cells combines oxygen and fuel (i.e., hydrogen gas) to give water, heat, and of course, electricity. A single fuel cell can only produce a small amount of power. However, multiple cells can be grouped in a fuel cell stack, allowing for the production of a high amount of energy.
Hydrogen fuel cells are an environmentally friendly option for powering engines. Since the only by-product is water, they are much cleaner than conventional internal combustion engines. Besides hydrogen, a variety of fuels can be used, such as natural gas and methanol. Fuel cells can operate for an infinite amount of time provided fuel is added.
Like the other batteries, fuel cell systems consist of anode and cathode, but also have an electrolyte membrane. The membrane is generally polymer-based (i.e., Nafion®), although there is a lot of commercial interest also in solid oxide fuel cells (SOFCs).
Conclusions
Since the development of the very first battery more than 200 years ago, electrochemistry has always had applications in real-life. Not only it finds uses in the building or naval sector by tackling corrosion, but it is also applied in other fields such as the development of sensors or metal deposition in jewelry manufacturing.
Enormous achievements have been made in the development of batteries and lots of research is still in progress, focussing on hot topics such as Li-ion batteries for portable devices and fuel cells for the production of clean energy in propulsion systems.
References
- Cifarelli, L., Mcevoy, A. J., Wagner, F. & Wiersma, D. S. (2013). Fundamentals and applications of electrochemistry. EPJ Web of Conferences, 54, 01018.10.1051/epjconf/20135401018
- Elewa, R. E., Afolalu, S. A. & Fayomi, O. S. I. (2019). Overview Production Process And Properties Of Galvanized Roofing Sheets. Journal of Physics: Conference Series, 1378, 022069.10.1088/1742-6596/1378/2/022069
- Manthiram, A. (2011). Materials Challenges and Opportunities of Lithium-Ion Batteries. The Journal of Physical Chemistry Letters, 2, 176-184.10.1021/jz1015422
- Sazali, N., Wan Salleh, W. N., Jamaludin, A. S. & Mhd Razali, M. N. (2020). New Perspectives on Fuel Cell Technology: A Brief Review. Membranes (Basel), 10.10.3390/membranes10050099
Further Reading
Last Updated: Jan 25, 2021