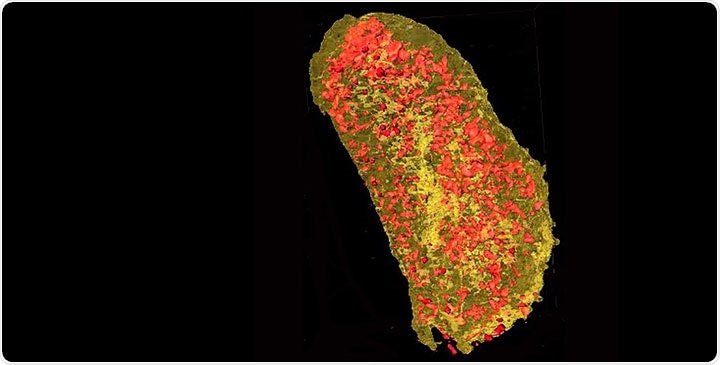
Ultrabright X-rays revealed the concentration of erbium (yellow) and zinc (red) in a single E. coli cell expressing a lanthanide-binding tag and incubated with erbium. Image Credit: Brookhaven National Laboratory.
Incidentally, NSLS-II is a U.S. Department of Energy (DOE) Office of Science User Facility at DOE’s Brookhaven National Laboratory.
In the structural biology world, scientists use techniques like X-ray crystallography and cryo-electron microscopy to learn about the precise structure of proteins and infer their functions, but we don’t learn where they function in a cell. If you’re studying a particular disease, you need to know if a protein is functioning in the wrong place or not at all.”
Lisa Miller, Study Corresponding Author and Scientist, National Synchrotron Light Source II
Created by Miller and her collaborators, the latest method has more or less the same approach as the conventional techniques of fluorescence microscopy that are used in biology, where a single molecule known as green fluorescent protein (GFP) can be adhered to other types of proteins to find out their location.
Upon exposing the GFP to visible or UV light, it fluoresces a radiant green color and lights up an otherwise “invisible” protein within the cell.
“Using GFP, we can see if a protein is in subcellular structures that are hundreds of nanometers in size, like the nucleus or the cytoplasm,” added Miller, “but structures like a cell membrane, which is only seven to 10 nanometers in size, are difficult to see with visible light tags like GFP. To see structures the size of 10 nanometers in a cell, you benefit greatly from the use of X-rays.”
Hence, to resolve this problem, the NSLS-II scientists collaborated with researchers from Boston University (BU) and the Massachusetts Institute of Technology (MIT), who successfully created an X-ray-sensitive tag known as a lanthanide-binding tag (LBT).
Being very small proteins, the LBTs can bind strongly to elements that belong to the lanthanide series, like europium and erbium.
Unlike GFP, which fluoresces when exposed to UV or visible light, lanthanides fluoresce in the presence of X-rays. And since lanthanides do not occur naturally in the cell, when we see them with the X-ray microscope, we know the location of our protein of interest.”
Tiffany Victor, Study Lead Author and Research Associate, National Synchrotron Light Source II
At NSLS-II, BU, and MIT, the research team worked together to integrate X-ray-fluorescence with LBT technology.
“Although LBTs have been used extensively over the last decade, they’ve never been used for X-ray fluorescence studies,” added Miller.
Apart from acquiring higher resolution images, X-ray fluorescence concurrently offers chemical images on all trace elements like zinc, copper, iron, potassium, and calcium present in a cell.
In other researches, Miller’s group is exploring how trace elements, such as copper, are associated with neuron death in Alzheimer’s diseases, for example.
Observing the presence of these elements with respect to certain proteins will be crucial to innovative discoveries. Apart from their compatibility with X-rays, LBTs are equally useful for their comparatively small size, as opposed to the visible light tags.
Imagine you had a tail attached to you that was the size of your whole body, or bigger. There would be a lot of normal activities that you’d no longer be able to do. But if you only had to walk around with a tiny pig’s tail, you could still run, jump, and fit through doorways. GFP is like the big tail—it can be a real impediment to the function of a many proteins. But these little lanthanide-binding tags are almost invisible.”
Lisa Miller, Study Corresponding Author and Scientist, National Synchrotron Light Source II
To show how the LBTs can be used for imaging proteins in 3D down to nanoscale resolution, the team at BU and MIT tagged a pair of proteins in a bacterial cell—one membrane protein and one cytoplasmic protein.
Miller’s team then analyzed the sample at the Bionanoprobe beamline based at the Advanced Photon Source (APS)—a DOE Office of Science User Facility at DOE’s Argonne National Laboratory—and at the Hard X-ray Nanoprobe (HXN) beamline based at NSLS-II.
“HXN offers the world-leading X-ray focus size, which goes down to about 12 nanometers. This was critical for imaging the bacterial cell in 3-D with nanoscale resolution,” stated Yong Chu, lead beamline scientist at HXN. “We also developed a new way of mounting the cells on a specialized sample holder in order to optimize the efficiency of the measurements.”
By combining the capabilities of LBTs with the unrivaled resolution of HXN, the researchers were able to precisely image the two tagged proteins.
The observation of the cell membrane protein demonstrated that the LBTs can be visualized at a high resolution, while imaging the cytoplasmic protein also demonstrated that LBTs could also be seen inside the cell.
“At high concentrations, lanthanides are toxic to cells,” added Victor, “so it was important for us to show that we could treat cells with a very low lanthanide concentration that was nontoxic and substantial enough to make it past the cell membrane and image the proteins we wanted to see.”
Now, armed with this latest method that was proven successful, the researchers are hoping to utilize the LBTs for imaging other types of proteins inside the cell at a resolution of 10 nm.
The research was financially supported by the National Science Foundation and the U.S. Department of Energy. The DOE’s Office of Science supports operations at APS and NSLS-II.
Source:
Journal reference:
Victor, T. W., et al. (2020) Lanthanide-Binding Tags for 3D X-ray Imaging of Proteins in Cells at Nanoscale Resolution. Journal of the American Chemical Society. doi.org/10.1021/jacs.9b11571.