Reviewed by Lexie CornerJul 9 2025
Scientists at Johns Hopkins Medicine have identified the location and function of energy-generating waves on the membranes of cancer cells. Their goal is to better understand how these cells support rapid growth and spread. The waves are driven by enzymes that convert glucose into energy.
According to the researchers, these waves could be useful for tumor staging and may serve as drug targets to slow or stop cancer progression.
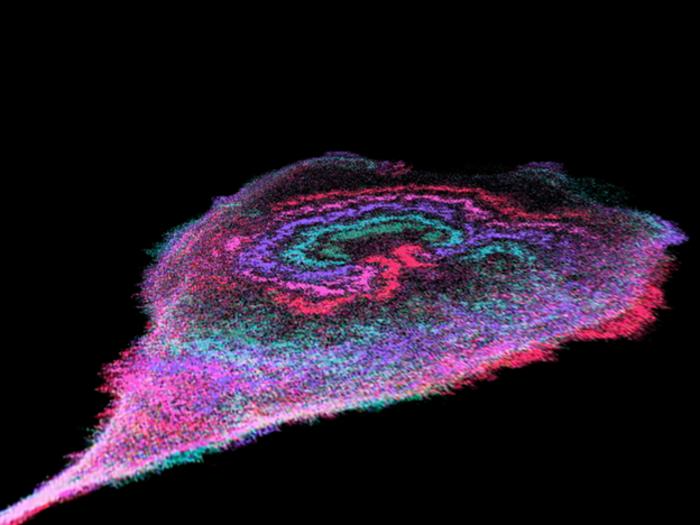 Glycolytic waves drive and fuel cancer cell migration. The image, captured with a high-powered microscope over 9-minute intervals, is color-coded from early to late time points, showing wave activity (green, blue, purple, pink, red). Image Credit: David Huiwang Zhan
Glycolytic waves drive and fuel cancer cell migration. The image, captured with a high-powered microscope over 9-minute intervals, is color-coded from early to late time points, showing wave activity (green, blue, purple, pink, red). Image Credit: David Huiwang Zhan
In lab experiments using human cancer cells, the researchers suggest that tracking these energy-producing waves could support more consistent cancer staging. This approach may work across various cancer subtypes and independent of genetic differences.
The findings were published on July 1, 2025, in Nature Communications. The study was partially funded by the National Institutes of Health.
Our findings suggest a correlation between higher levels of the energy-producing waves and a greater severity of the cancer, or the cancer’s potential to spread to other organs.
Peter Devreotes, Ph.D., Isaac Morris and Lucille Elizabeth Hay Professor, Johns Hopkins University School of Medicine
In cancer biology, the Warburg effect has long been recognized. This effect refers to cancer cells' preference for glycolysis - an inefficient energy production process - over oxidative phosphorylation, which is more efficient.
That appears to be a paradox for cancer because cancer cells need much more energy to grow than normal cells.
David Zhan, Ph.D., Postdoctoral Researcher, Johns Hopkins University School of Medicine
The researchers noted that glycolysis was traditionally thought to occur uniformly in the cell's cytosol. However, in their analysis of lab-grown cancer cells, they observed that glycolytic enzymes cluster and move in wave-like patterns along the cell membrane. This indicates a more organized process of energy production.
“This finding may challenge the canonical textbook knowledge that we all learn from the biochemistry course,” Zhan remarked.
To begin the study, Zhan and colleagues compared normal human breast duct cells with breast cancer cells. They used genetic engineering to attach fluorescent markers to glycolytic enzymes, allowing detailed observation under a high-resolution microscope.
They found large amounts of glycolytic enzymes on the membranes of breast cancer cells. These enzymes moved in ordered waves. In contrast, the researchers observed very few glycolytic enzymes or wave patterns on normal cells.
“The more aggressive the cancer, the more waves we found on the cell surface,” Devreotes noted.
This work builds on a 2020 study published in Developmental Cell by Zhan and Devreotes, which also linked glycolytic wave activity with cancer progression.
The current study measured ATP levels in breast cancer cells and normal cells. More aggressive cancer subtypes showed higher ATP production linked to glycolytic wave activity.
Similar results were found in other cancer cell types, including pancreatic, lung, breast, colon, and liver cancers. In each case, more aggressive subtypes showed higher ATP levels and greater wave activity compared to less aggressive ones.
Zhan further added, “The increased presence of these glycolytic waves drives more ATP production from glycolysis in cancer cells, and that leads to enhanced reliance on glycolysis for energy.”
o test whether disrupting the waves could reduce energy production, the researchers used Latrunculin A (LatA), a small molecule that interferes with wave formation. After treatment, they observed a 25 % reduction in ATP levels, suggesting that the waves play a key role in maintaining the energy supply of cancer cells.
“When we inhibit the activity of these waves, we may be able to stop these cancer cells from being able to consume nutrients and grow. Cancer cells require a lot of energy to drive cancer malignancy, so disrupting this process might be able to slow or stop its spread,” Zhan noted.
Devreotes stated that the team’s next step is to investigate the exact mechanism by which these energy waves form on the cell membrane.
Source:
Journal reference:
Zhan, H., et al. (2025) Self-organizing glycolytic waves tune cellular metabolic states and fuel cancer progression. Nature Communications. doi.org/10.1038/s41467-025-60596-6.