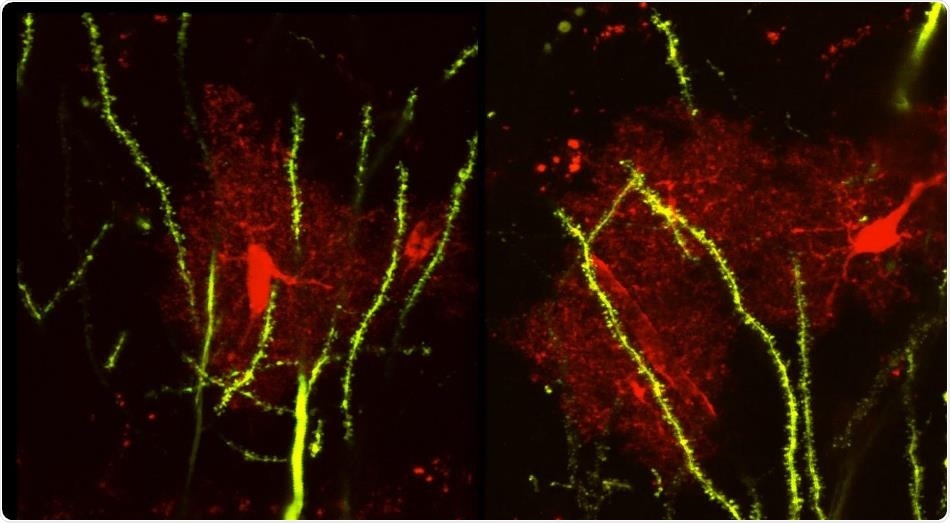
Confocal image of a mouse brain tissue shows the astrocytes (red) and neurons (green). Image Credit: University of California, Riverside/Ethell laboratory.
The team targeted the brain region known as hippocampus—which plays a significant role in social interactions and learning—and synapses, which are essentially specialized contacts found between neurons.
Within the brain, each neuron receives various inhibitory and excitatory synaptic inputs. The balance between inhibition and excitation in neuronal circuits—called E/I balance and believed to be significant for data processing in the central nervous system and crucial for circuit stability and function—can contribute to several neurological disorders, such as autism spectrum disorder, epilepsy, and schizophrenia.
The team also targeted a protein, known as ephrin-B1, which covers the membrane around the cell and plays a key role in preserving the nervous system.
The aim of the researchers’ study was to find out whether the over-production or deletion of the ephrin-B1 protein in astrocytes—which are glial cells in the brain that control the synaptic connections within neurons—influences the formation and maturation of synapses in the developing hippocampus and modifies the E/I balance, resulting in behavioral deficits.
We found the changes in the E/I balance are regulated by astrocytes in the developing brain through the ephrin protein. Further, astrocytic ephrin-B1 is linked to the development of inhibitory networks in the hippocampus during a critical developmental period, which is a new and unexpected discovery.”
Iryna Ethell, Professor of Biomedical Sciences, School of Medicine, University of California, Riverside
Ethell, who also headed the mouse study, added, “Specifically, we show the loss of astrocytic ephrin-B1 tilts the E/I balance in favor of excitation by reducing inhibition, which then hyperactivates the neuronal circuits. This hyperactivity manifests as reduced sociability in the mice and suggests they can serve as a new model to study autism spectrum disorder.”
Published in The Journal of Neuroscience, the discoveries can improve scientists’ interpretation of the mechanisms that result in neurodevelopmental disorders. Such an insight would allow them to identify new interventions for treating such disorders by focusing astrocytes during a certain developmental period.
Ethell further described that the dysfunctions of astrocytes are also associated with synapse pathologies linked to neurodegenerative diseases and neurodevelopmental disorders, like Alzheimer’s disease, in which neuron loss can also occur due to early dysfunction in synaptic connections.
Ethell added, “How exactly astrocytes use the ephrin protein to control the development of neuronal networks remains to be explored in future studies. Our findings open a new inquiry into future clinical applications as impaired inhibition has been linked to several developmental disorders, including autism and epilepsy.”
This is the first report to establish a connection between astrocytes and the development of E/I balance detected in the mouse hippocampus at the time of early postnatal development.
We provide new evidence that different ephrin-B1 levels in astrocytes influence both excitatory and inhibitory synapses during development and contribute to the formation of neuronal networks in the brain and associated behaviors.”
Iryna Ethell, Professor of Biomedical Sciences, School of Medicine, University of California, Riverside
Ethell described that synapses are essentially building components of neural networks and work as basic data-processing units in the brain. She further added that excitatory synapses are essentially cell-cell connections that enable neuronal activity, while inhibitory connections negatively control brain activity to synchronize the responses, specificity, and timing of the brain.
Ethell stated, “Hyperactivity of neuronal networks resulting from the loss or impaired function of inhibitory synapses can lead to neural dysfunctions and seizures. Like a car without brakes, the brain without inhibitory neurons cannot function properly and becomes overactive, resulting in loss of body control.”
She agreed that additional studies are required to find out how ephrin signaling in astrocytes precisely changes the inhibitory synapses, and particularly how astrocytes could play a role in such mechanisms.
Given the widespread and growing research interest in the astrocyte-mediated mechanisms that regulate E/I balance in neurodevelopmental disorders, our findings establish a foundation for future studies of astrocytes in clinically relevant conditions.”
Iryna Ethell, Professor of Biomedical Sciences, School of Medicine, University of California, Riverside
Source:
Journal reference:
Nguyen, A. Q., et al. (2020) Astrocytic ephrin-B1 controls excitatory-inhibitory balance in developing hippocampus. The Journal of Neuroscience. doi.org/10.1523/JNEUROSCI.0413-20.2020.