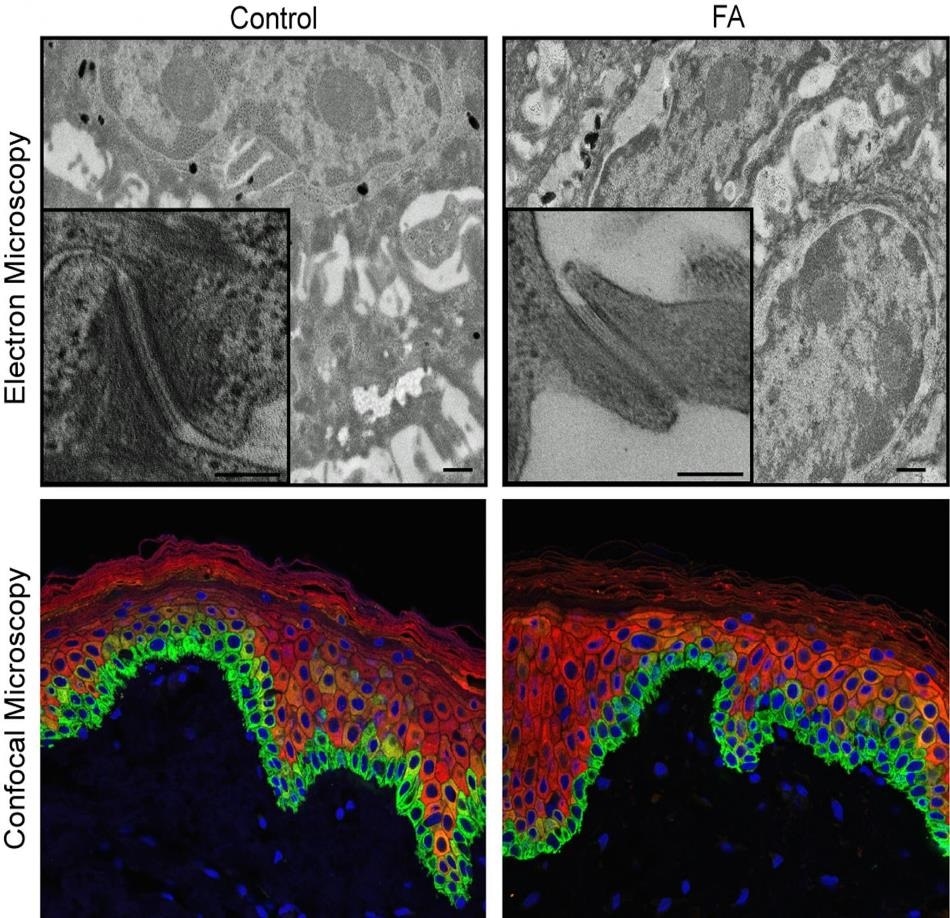
Shown are microscopic images of the human epidermis with pluripotent stem cells derived from donated skin cells. The images on the left are epidermis from a healthy control subject, the images at right being from a person with Fanconi anemia. The colorful confocal images (bottom) offer a more superficial view that does not reveal differences between control and FA samples. The black and white electron microscopic images, with 1,000-fold greater magnification, do reveal defects in the FA epidermis. Researchers studying Fanconi anemia-related skin disease and cancer report new data in Cell Stem Cell. Image Credit: Cincinnati Children’s Hospital Medical Center.
This approach allowed the team to closely and precisely visualize how inherited defects in the DNA cause skin damage as well as lethal squamous cell carcinoma in young adults and children suffering from Fanconi anemia (FA).
The authors of the study explained that the new tissue models derived from human stem cells resolve the intrinsic restrictions when it comes to analyzing human disease in mice. This gives scientists a novel tool to ultimately resolve a dangerous and long-standing molecular enigma.
Squamous cell carcinoma is a global health problem, and DNA instability in children with Fanconi anemia makes them extremely susceptible. Unlike the general population, squamous cell carcinomas that arise in the head, neck, anogenital regions, and skin of children and young adults with FA tend to be unusually aggressive and deadly.”
Susanne Wells, PhD, Study Principal Investigator and Cancer Biologist, Cancer and Blood Diseases Institute, Cincinnati Children’s Hospital Medical Center
Although treatments are available for FA, Wells informed that such therapies involve side effects because of the workings of the disease.
We need effective treatments, but identifying the molecular and cellular consequences of FA gene mutations has been difficult because mouse models don’t fully recapitulate human disease. Fortunately, our bioengineered models of 3D human epidermis are helping us overcome this.”
Susanne Wells, PhD, Study Principal Investigator and Cancer Biologist, Cancer and Blood Diseases Institute, Cincinnati Children’s Hospital Medical Center
Wells is also the director of the Epithelial Carcinogenesis and Stem Cell Program.
A pathway to DNA instability
FA is known to be an inherited disorder and occurs when there is a loss of function mutations in more than genes in human reproductive, or germline, cells. The FA pathway often plays a crucial role in the structure and function of normal skin.
While all cells contain cross-linked DNA, faulty DNA repair machinery in patients suffering from FA condition leads to the build-up of defective crosslinks. As a result, children with FA are prone to bone marrow failure, DNA instability, and cancer.
In the current study, scientists demonstrated this crucial role in their latest data. They performed a small controlled clinical test to prove that patients who have FA mutations are more likely to experience skin damage and blistering induced by environmental stress.
The Institutional Review Board at the Cincinnati Children Hospital Medical Center has approved the test, in which moderate pressure is applied to the arms of young adults and children suffering from FA and also to a control group without the FA condition.
Patients with FA developed skin blisters relatively faster when compared to those in the non-FA control group, indicating inherent skin fragility in this set of populations.
Mimicking nature’s developmental process
To monitor the biological development of epidermal susceptibilities in kids with the FA condition, the researchers used donated skin tissue to create patient-derived pluripotent stem cells (PSCs). These PSCs adopt embryonic-like characteristics and can create any type of tissue in the body.
FA gene mutations were observed in the patient-specific stem cells used in this research work. For direct comparison, these FA gene mutations could be rectified by scientists through an inducible system.
Subsequently, the PSCs were biochemically transformed into progenitor and epidermal stem cells—the developmental phase at which FA mutations typically set out to disrupt the function of the skin.
Progenitor cells and epidermal stem cells were subsequently used to create complex 3D epidermal models, known as organotypic skin rafts. These skin rafts also exhibited FA mutations when they were left untreated.
The FA patient-specific tissues had reduced cell-to-cell junctions—major biological connections that are crucial for the formation and function of the skin, along with other structural and molecular flaws. Such defects increased the rate of blistering of skin following mechanically induced stress, which triggers the processes of disease that can develop into cancer.
Skin fragility in patients with the FA condition may also promote cancer when the body is increasingly exposed to carcinogens in the external surroundings.
According to Sonya Ruiz-Torres, Ph.D., the study’s first author and a fellow in Wells’ laboratory, the team is continuing their study. Since the research work was restricted by a few number of patients, the investigators are creating 3D human organotypic skin rafts to analyze a wider range of individuals with FA mutations.
This strategy should give researchers a more detailed look at varied processes of FA gene mutations, figure out how these encourage squamous cell carcinoma and help improve the promising clinical impact of their study.
Source:
Journal reference:
Ruiz-Torres, S., et al. (2020) Inherited DNA Repair Defects Disrupt the Structure and Function of Human Skin. Cell Stem Cell. doi.org/10.1016/j.stem.2020.10.012.