Researchers are paving the way to a more in-depth understanding of the continuously shifting evolutionary arms race between pathogens and the host organisms they search to infect. Pathogens and host organisms are in a persistent chess match to manipulate the weaknesses of each other.
A new study by the University of California - San Diego biologists has revealed insights on the intricate, adaptive mechanisms of a protective system employed by the cells of mammalian immune systems. These defenses have evolved to set a type of tripwire that produces an immune response against attacks from viruses. Image Credit: University of California, San Diego.
This study provides exciting clues on human health, considering that the immune system is on continuous alert to use countermeasures against the attacks of new viruses, However, releasing excess defensive response can result in self-inflicted damage to tissues and various diseases.
Now, a new study has shed light on the complex, adaptive mechanisms of a protective system used by the cells of mammalian immune systems. The study was published in the eLife journal by biologists from the University of California, San Diego.
Using a multidisciplinary technique that integrated virology, biochemistry, and bioinformatics, three Biological Sciences graduate students, including Brian Tsu, Chris Beierschmitt, and Andy Ryan, Assistant Professor Matt Daugherty, and their colleagues from UC Berkeley discovered unexpected defensive functions mediated by a protein known as NLRP1, which acts as a sensor for invading pathogens.
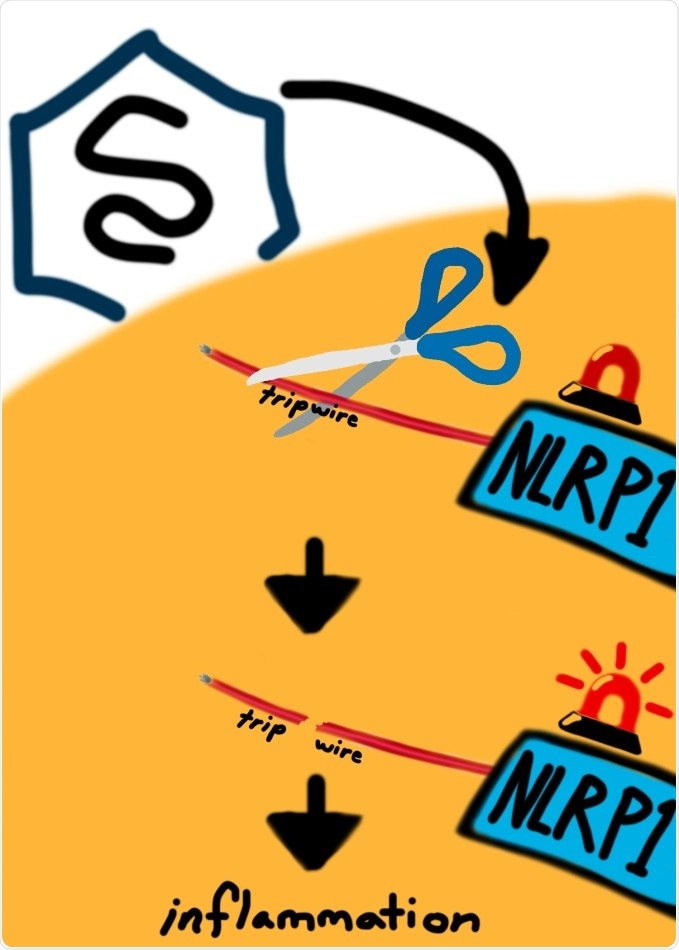
The research work included Picornaviridae family viruses, which produce molecular “scissors,” or proteases, that can cleave and stimulate the NLRP1 protein. Such viruses include human pathogens, like rhinovirus (one of the most common causes of the common cold), coxsackievirus (that causes foot, hand, and mouth disease), and poliovirus.
The study demonstrated that the NLRP1 protein has lately evolved to “sense” such viral proteases via a type of trap that triggersan immune reaction in response to being cut by these viral proteases.
Fascinatingly, the NLRP1 protein has evolved to perform this activity by imitating natural locations that are usually required by the viral protease to cut, so that the virus to replicate. This makes it hard for the pathogen to avoid cleaving the NLRP1 protein while still retaining its potential to live.
In our paper we're showing that NLRP1 acts to bait viral protease cleavage and set off a sort of alarm, or tripwire, in the organism. This is like an Achilles heel to the virus. This allows the host organism to evolve ways to take advantage of this evolutionarily constrained cleavage.”
Brian Tsu, Study Lead Author, University of California, San Diego
According to Daugherty, the results provide an exciting switch of traditional views about the dynamics of virus and host.
We often think of viruses taking advantage of the fact that hosts evolve slowly, but we're seeing that the hosts have turned the tables and used the fact that the viruses are really stuck here to their advantage, and therefore they use this constraint to activate an immune response.”
Matt Daugherty, Assistant Professor, University of California, San Diego
Although evolution is usually believed to occur in a sequential step, the viruses examined in this analysis would need to concurrently modify multiple regions inside their viral proteins to emerge around the tripwire defense, which would be very complicated.
While the study was derived in cells, it lays the basis for possible upcoming clinical applications, where the tripwire function could be used in immune defenses in human systems, for example, the brain, lungs, and other regions. Depending on the study results in individual cells, innovative research avenues are paving the way to study how the tripwire works across whole organisms.
I'm particularly excited about looking for more of these cases because this is an evolutionarily elegant way of detecting and responding to viral infection.”
Matt Daugherty, Assistant Professor, University of California, San Diego
Source:
Journal reference:
Tsu, B. V., et al. (2021) Diverse viral proteases activate the NLRP1 inflammasome. eLife. doi.org/10.7554/eLife.60609.