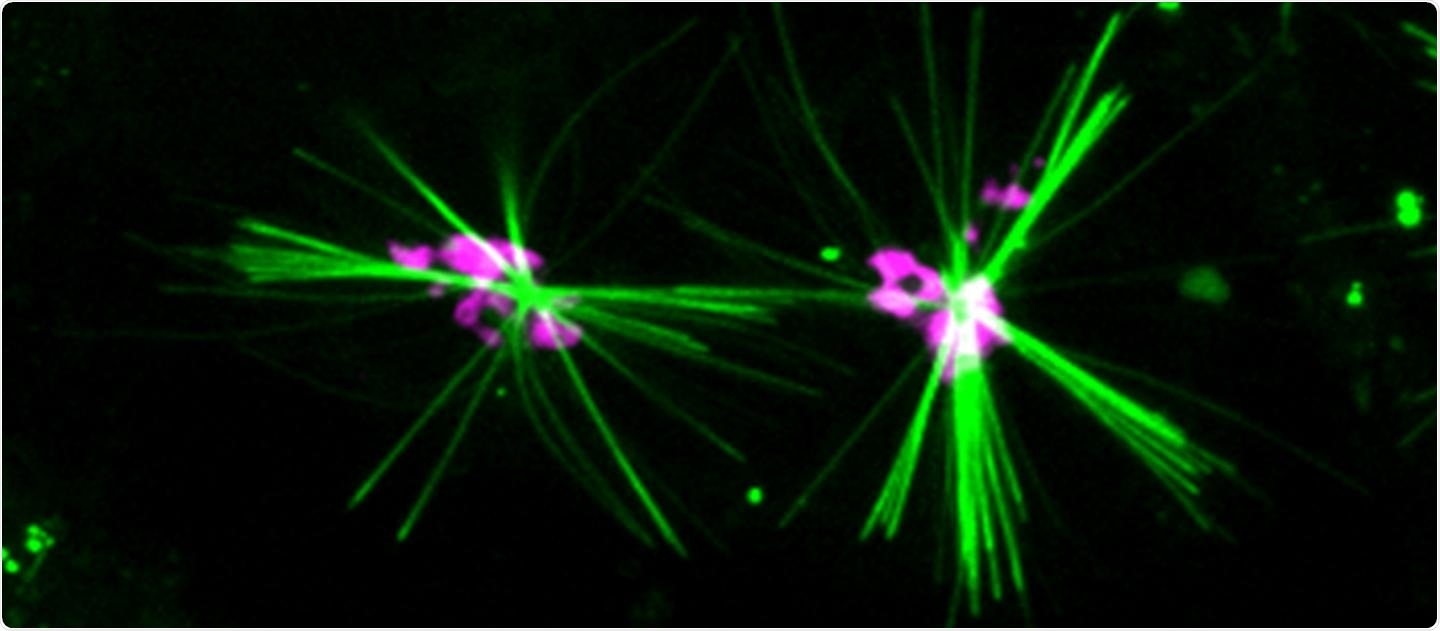
A precipitating dye generates fluorescent, aster-like crystals (green) in live cells recording the motion of the motor protein kinesin-1 along microtubules. The crystals are centered in the Golgi apparatus (magenta) and extend toward the periphery of the cells, consistently with the transport activity of kinesin-1. Image Credit: © University of Geneva.
But researchers are clueless as to how do the proteins that transport these cargoes—the kinesins—are able to locate their path and direction inside the “road network” of the cell to deliver them to the correct place.
It all started from a research that didn't go as planned. Initially, we wanted to develop a molecule that would make it possible to visualize the stress level of the cell, i.e. when it accumulates too much active oxygen species. During the experiment, the molecule did not work, but crystallized. Why did it crystallize? What were these crystals?”
Nicolas Winssinger, Professor, Department of Organic Chemistry of the Faculty of Science, University of Geneva
Three theories appeared to be possible and the researchers sought the help of Charlotte Aumeier, a professor in the Department of Biochemistry of the Faculty of Sciences of the UNIGE to confirm them. The first theory indicated that crystallization was caused by the microtubules that polymerize.
Aumeier explained, “Microtubules are small, rigid tubes that can grow or shrink and constitute the “road network” that allows molecules to move around the cell.”
The second theory made the Golgi apparatus accountable for this chemical reaction. And the third theory indicated that the crystals were the outcome of the tiny steps made by the kinesin proteins in the microtubules as they circulated inside the cell.
The biochemists' little thumb
To confirm these various options, the UNIGE research team collaborated with the National Institute of Health (NIH) in Bethesda (United States), which deals in electron microscopy.
We first recreated microtubules that we purified, which takes 14 hours. For the kinesins, the motor proteins that move on microtubules and transport cargo, we isolated them from bacteria.”
Charlotte Aumeier, Professor, Department of Biochemistry of the Faculty of Sciences, University of Geneva
The team then assembled around 20 various mixtures comprising the small molecule QPD, which is methodically found in the crystals and noted which solution actually worked.
“We wanted to know what was needed to form the crystals. The microtubules? The kinesin? Yet another protein?,” asked Winssinger.
After different experiments, the researchers found that the formation of such crystals was induced by one of the 45 types of kinesin found in the cell. The Geneva-based researcher added, “With each small step that this kinesin protein takes on the microtubule, it uses energy that leaves a trace identified by the QPD molecule.”
The crystals are formed from this recognition. In this manner, the crystals are chemically left behind by the kinesin passage, and researchers can track this kinesin similar to a small thumb.
The opening of a new field of study
Until now, it has not been possible to track a particular protein. With current techniques, we couldn't separate the individual kinesins, so we couldn't see which path they took precisely. Thanks to the development of our new chemical fluorescent dye, we can observe in detail how a protein behaves, which route it takes, its direction or even its preferred path.”
Charlotte Aumeier, Professor, Department of Biochemistry of the Faculty of Sciences, University of Geneva
For the first time, researchers can envision the walking track of motor proteins and investigate the underlying question of the transport activity and also the distribution of cargoes within the cells.
Source:
Journal reference:
Angerani, S., et al. (2021) Kinesin-1 activity recorded in living cells with a precipitating dye. Nature Communications. doi.org/10.1038/s41467-021-21626-1.