Cell-cell adhesion molecules, which link opposing cells, preserve the structure of the cell, tissue, and organ. Various diseases are driven by deficiencies in cadherin function, which is a family of crucial cell-cell adhesion molecules for tissue development and integrity (e.g., cancer invasion). In order to facilitate cell-cell adhesion, cadherin extends from the cell surface and adheres to another cadherin on an opposing cell.
 HS-AFM images of the cadherin dimers are shown at the top. The binding mechanism of cadherins is illustrated at the bottom based on HS-AFM observations. Image Credit: Shigetaka Nishiguchi of Scientists at the Exploratory Research Center on Life and Living Systems (ExCELLS).
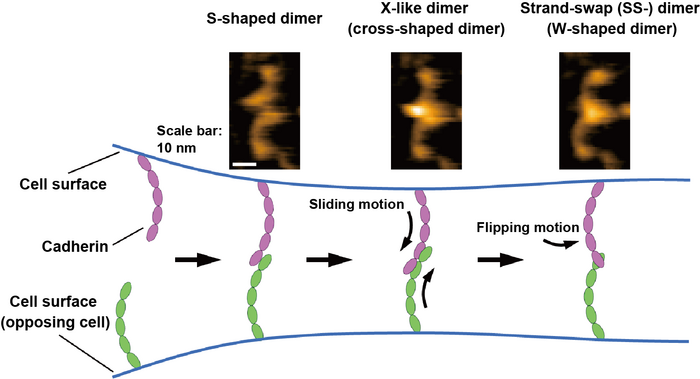
HS-AFM images of the cadherin dimers are shown at the top. The binding mechanism of cadherins is illustrated at the bottom based on HS-AFM observations. Image Credit: Shigetaka Nishiguchi of Scientists at the Exploratory Research Center on Life and Living Systems (ExCELLS).
The two dimerization processes that make up the cadherin binding process are the generation of X-dimers and strand-swap (SS-) dimers of the extracellular domains (ectodomains) of cadherin. The specific binding mechanism of cadherin, however, is still up for debate. Other interactions than those involving the creation of the X- and SS-dimers have also been postulated.
High-speed atomic force microscopy (HS-AFM) was used by Takayuki Uchihashi of ExCELLS, Shigetaka Nishiguchi of ExCELLS, Nagoya University, and Tadaomi Furuta of Tokyo Tech to investigate the binding mechanism of cadherins.
By physically contacting and scanning the surface of proteins with a fine-tipped probe, HS-AFM may allow the observation of single-molecule structures and dynamics in solution at the nanometer scale with sub-second temporal resolution. The existence of cadherins as numerous dimer configurations, which may be categorized as W-, cross-, and S-shaped dimers depending on their morphology, was discovered using HS-AFM.
The researchers also performed mutational and structural modeling investigations and discovered that the S-shaped dimer is a new conformation and that W- and cross-shaped dimers are related to recognizing SS-dimers and X-like dimers.
Through conversion into the three different forms of dimeric structures indicated above, the cadherin binding processes are directly seen by HS-AFM further demonstrating that the dimerization process is finished in less than one second.
Based on these HS-AFM findings, the researchers proposed that the binding process progresses by sliding motion of the S-shaped dimer, followed by a flipping motion of the X-dimer to generate the SS-dimer, which is believed to be the ultimate stable cadherin dimer seen in Figure.
Since structural studies and measurements of cells and solutions can only be used to evaluate the binding states represented by the many cadherins, they have mostly been used to explore the binding mechanism of cadherins up to this point.
The newly developed HS-AFM approach, which has not before been accomplished, showed the binding mechanisms of certain cadherins at single-molecule resolution. HS-AFM observation will open the door to a better comprehension of cadherins’ binding mechanism, which is crucial for tissue and organ organization and diseases connected to cell-cell adhesion.