Scientists at Weill Cornell Medicine have created a computational method for mapping the architecture of human tissues in extraordinary detail. Their method guarantees to expedite research on organ-scale cellular interactions and could lead to strong new diagnostic strategies for a variety of disorders.
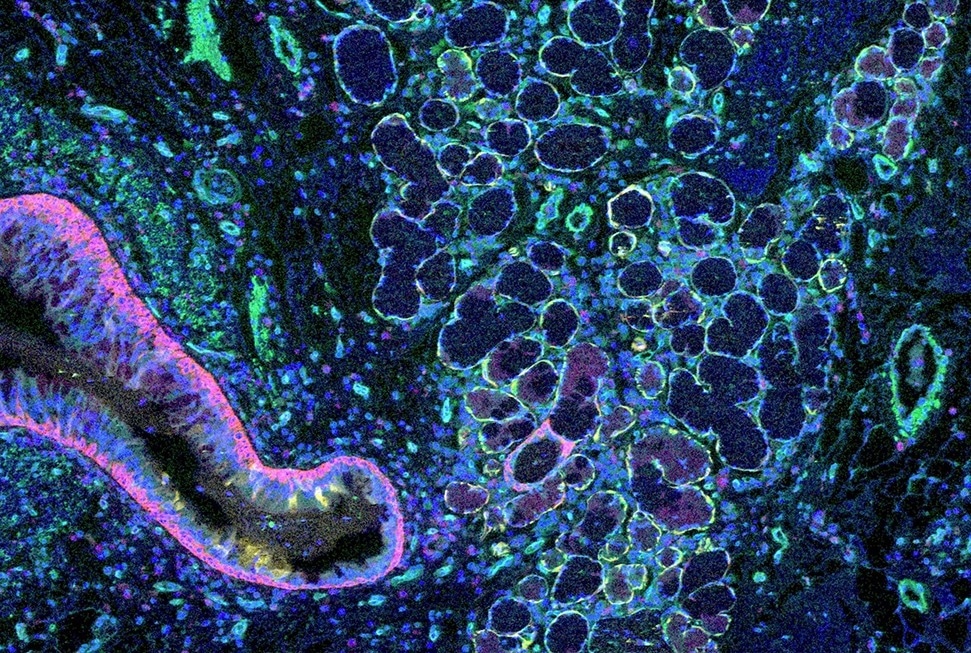 Mass cytometry image of a healthy lung. The red structure on the left is an airway; the yellow round structures in middle are submucosal glands, and the small round green structures are blood vessels. Image Credit: Junbum Kim.
Mass cytometry image of a healthy lung. The red structure on the left is an airway; the yellow round structures in middle are submucosal glands, and the small round green structures are blood vessels. Image Credit: Junbum Kim.
The approach, which was published in Nature Methods on October 31st, 2022, resulted from the scientists’ dissatisfaction with the gap between classical microscopy and modern single-cell molecular research.
Looking at tissues under the microscope, you see a bunch of cells that are grouped together spatially—you see that organization in images almost immediately. Now, cell biologists have gained the ability to examine individual cells in tremendous detail, down to which genes each cell is expressing, so they’re focused on the cells instead of focusing on the tissue structure.”
Junbum Kim, Study Lead Author and Graduate Student, Physiology and Biophysics, Weill Cornell Medicine
According to senior author Dr Olivier Elemento, Director of the Englander Institute for Precision Medicine and a Professor of Physiology and Biophysics and of Computational Genomics in Computational Biomedicine at Weill Cornell Medicine, “it’s crucial for researchers to learn more about the details of tissue structure; fundamental changes in the relationships between cells within a tissue drive both healthy and diseased organ function.”
Manually merging single cell data with tissue structure maps, on the other hand, is time-consuming and inefficient. Machine learning algorithms have demonstrated some promise for automating the procedure, but their abilities are restricted by the data needed to train them.
To address this, Kim and his colleagues devised an unsupervised computational approach that defines structural regions inside a tissue by combining single-cell gene expression profiles with cell locations.
Dr André Rendeiro, who was a postdoctoral fellow at Weill Cornell Medicine during the research and is now a principal investigator at the Austrian Academy of Sciences’ Research Center for Molecular Medicine in Vienna, Austria, contrasts the new approach to mapping a city like New York:
One way to go about it would be to go to every intersection and count each kind of building: is it residential, is it commercial … is it a shop or restaurant?”
Dr André Rendeiro, Principal Investigator, Research Center for Molecular Medicine, Austrian Academy of Sciences
After entering all of the relevant information into one matrix and the locations of the buildings into another, one might merge the two matrices and search for patterns.
Essentially, we could start to make a general statement about where the different neighborhoods are and where their borders are based on the abundance of, say, residential versus commercial buildings—just as anyone walking through the Upper East Side, Midtown or Downtown would do based on their observations.”
Dr André Rendeiro, Principal Investigator, Research Center for Molecular Medicine, Austrian Academy of Sciences
The new approach was used by the investigators to create precise maps of various types of tissues, detecting and characterizing new features of microanatomy—the patterns that arise at a small scale when cells interact and affect the eventual function of the tissue.
They also proved, in collaboration with a colleague at the UNC School of Medicine who researches lung disease, that their approach could create precise shades of differentiation between various disease states in a tissue.
Although cancer and other chronic disorders frequently result in significant changes in tissue structure, thorough microanatomy may also aid in the identification and management of more severe conditions. Rendeiro cites severe COVID-19 as an instance, stating that, “there are a lot of immune cells that move into the neighborhood, and there’s really dramatic change in the lung tissue.”
The researchers are now using their innovative technique on a variety of tissues to better understand how alterations in tissue organization underpin tissue function in health and dysfunction in disease.
Source:
Journal reference:
Kim, J., et al. (2022) Unsupervised discovery of tissue architecture in multiplexed imaging. Nature Methods. doi.org/10.1038/s41592-022-01657-2.