Researchers from The University of Texas MD Anderson Cancer Center have described a previously unrecognized type of cell death called disulfidptosis in a study that was published in Nature Cell Biology. This discovery could pave the way for novel cancer therapeutic approaches.
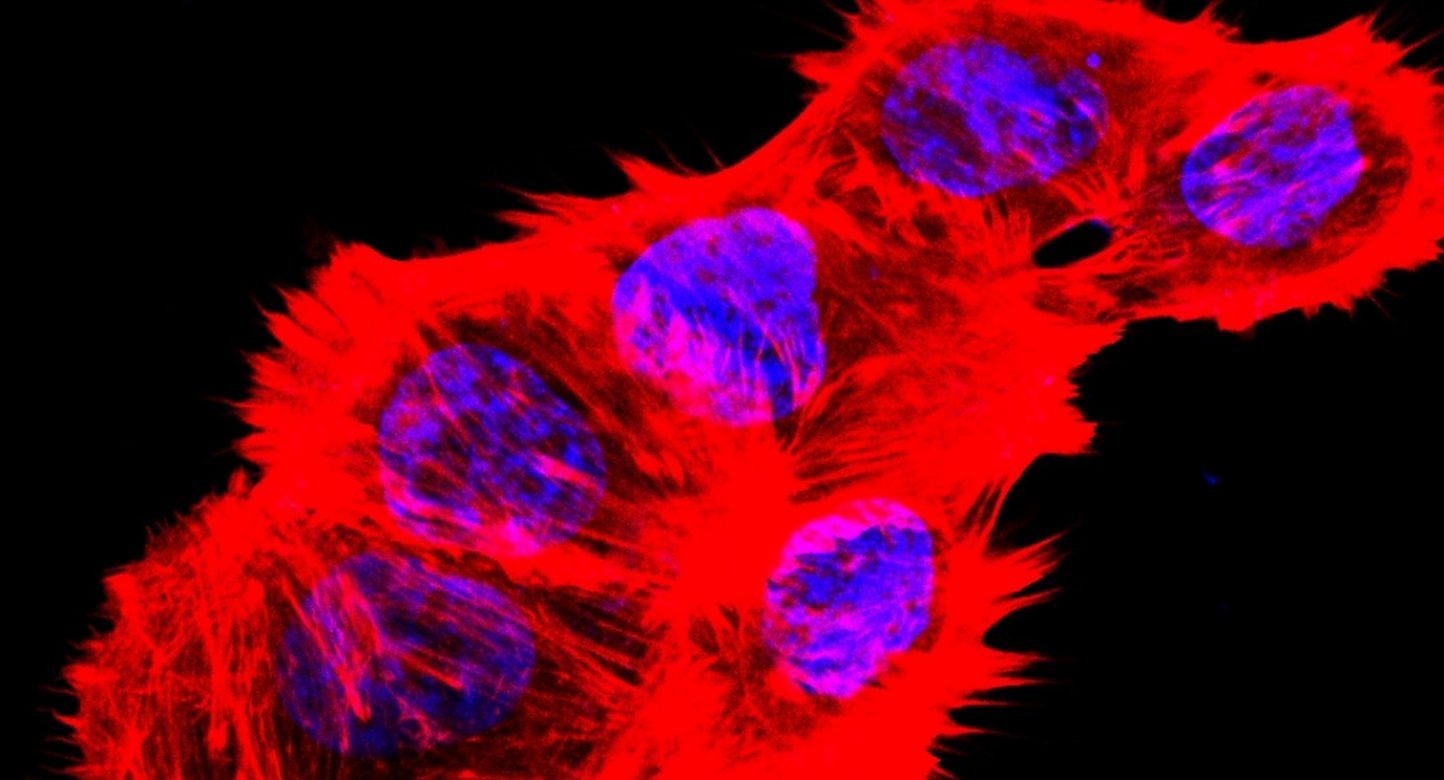 Image of F-actin and nucleus of human renal carcinoma cancer cells cultured with glucose-free medium. Image Credit: Xiaoguang Liu, PhD
Image of F-actin and nucleus of human renal carcinoma cancer cells cultured with glucose-free medium. Image Credit: Xiaoguang Liu, PhD
According to the study, glucose deprivation causes cells with high amounts of the SLC7A11 protein to undergo disulfidptosis. Treatment with glucose inhibitors in preclinical models led to the induction of disulfidptosis in cancer cells with high SLC7A11 expression, substantially inhibiting tumor growth without causing considerable damage to healthy organs.
Boyi Gan, PhD, and Junjie Chen, PhD, both professors of Experimental Radiation Oncology, were in charge of the study.
Cancer cells rely on SLC7A11 to import cystine for maintaining redox balance and for cell survival. However, this also exposes an Achilles heel in SLC7A11-high cancer cells because these cells are dependent on glucose to resolve their disulfide-overloading issue. Starving these cells of glucose can overwhelm them with toxic disulfide molecules, resulting in rapid cell death.”
Boyi Gan, PhD, Professor, Department of Experimental Radiation Oncology, Division of Radiation Oncology, The University of Texas MD Anderson Cancer Center
SLC7A11, which codes for the cystine transporter, is overexpressed in several malignancies, including lung and kidney cancer. The high expression of SLC7A11 and the ensuing “addiction” to extracellular glucose demonstrated by Gan’s team in a 2020 study suggested that some cancer cells could be amenable to therapy with glucose transporter inhibitors.
Cysteine is a crucial amino acid that the SLC7A11 protein imports, however cystine and other disulfide molecules can be hazardous in high concentrations. Cells are compelled to employ the chemical NADPH to swiftly change hazardous disulfides into other non-toxic molecules to maintain this equilibrium.
Since glucose is the primary source of NADPH, shutting off the glucose supply can cause a buildup of disulfide molecules and eventual cell death.
Up until this, it was unclear exactly how this procedure worked. Gan claims that by showcasing a type of cell death that has not yet been identified, this new study offers insight into the subject.
Apoptosis is one of the most well-known methods of cell death. It can be initiated either internally or externally and causes the activation of caspases, which destroy the cell by slicing apart vital proteins. Ferroptosis, which is brought on by the buildup of lipid peroxides, is a further mechanism of cell death that has received a lot of attention in recent years.
Disulfidptosis differs from these other methods of cell death because it involves the actin cytoskeleton, which is essential for preserving cell shape and survival. Actin filaments make up the actin cytoskeleton, which gives cells their general form and structure.
This recent study showed that the abundance of accumulated disulfide molecules causes abnormal disulfide bonding among actin cytoskeleton proteins in glucose-starved SLC7A11-high cancer cells, interfering with their structure and eventually resulting in actin network collapse and cell death.
Many cancer treatments aim to eradicate cancer cells by apoptosis. However, a lot of cancer cells discover methods to avoid the apoptosis that therapy causes, resulting in therapy resistance and disease return. These results imply that targeting disulfidptosis as a cancer therapy strategy needs more research.
Gan added, “This important finding will hopefully inspire disulfidptosis-inducing treatments for cancers that have evaded other therapies and are resistant to apoptosis. Because SLC7A11 is highly expressed in many cancers, there might be a therapeutic window to inhibit glucose transporters and induce disulfidptosis in these cells while leaving normal cells unaffected.”
The next step in this research, according to Gan, entails figuring out how other situations might cause disulfidptosis and what other pathways are involved. A deeper comprehension of these pathways could provide new cancer treatment targets.
Source:
Journal reference:
Liu, X., et al. (2023). Actin cytoskeleton vulnerability to disulfide stress mediates disulfidptosis. Nature Cell Biology. doi.org/10.1038/s41556-023-01091-2