Reviewed by Danielle Ellis, B.Sc.Apr 28 2023
CRISPR achieved scientific popularity for its capacity to quickly and precisely edit genes. At the core, however, CRISPR systems are immune systems that aid bacteria to safeguard themselves from viruses through targeting and abolishing viral RNA and DNA.
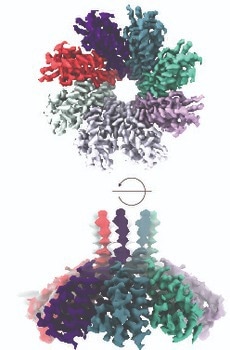
Csx28 membrane protein. Image credit: University of Rochester Medical Center
Science published the new research that shows a previously unrecognized player in one of those systems—a membrane protein that improves anti-viral defense.
Uncovering New Clues about CRISPR
CRISPR systems comprise two key components—a guide RNA that targets a particular viral RNA or DNA sequence and a Cas enzyme that cuts the targeted RNA or DNA, avoiding a virus from spreading and replicating. A research group at the University of Rochester Center for RNA Biology discovered that a particular Cas protein (Cas13b) not only cuts viral RNA but interacts with another protein (Csx28) to improve its anti-viral defense.
In collaboration with researchers at Cornell, the team found that the Csx28 protein creates a pore-like structure (implying that it has a huge hole in it). When E. coli is infected with a phage (a virus that attacks bacteria) and deployed the CRISPR-Cas13 system to target and halt infection, they discovered that Cas13 signals to Csx28 to cause impacts on membrane permeability.
Image Credit: ART-ur/Shutterstock.com
After this, Csx28 causes chaos in the infected cell, discombobulating the potential of the membrane, crushing metabolism, and hampering energy production. A virus cannot replicate in those unhospitable conditions, resulting in the team’s inference that Csx28 improves phage defense of CRISPR-Cas13b.
This finding upends the idea that CRISPR systems mount their defense only by degrading RNA and DNA in cells and really broadens our view of how CRISPR systems may be working. When we think about CRISPR, we see Cas proteins such as Cas9 or Cas13 as the big hammer doing all the damage, but that might not be the case; we found that Cas13 and Csx28 are working together to effectively extinguish a virus.”
Mitchell O’Connell, PhD, Corresponding Author and Assistant Professor, Biochemistry and Biophysics, University of Rochester Medical Center
He is also a member of the UR Center for RNA Biology.
When you read this paper you think to yourself…‘what?’ This is such a weird mechanism and not the way I would have predicted that bacteria would work. It is really impressive that the team identified this pore-like protein that doesn’t resemble anything else we’ve seen before, and now that we know that this mechanism exists people will start to look for it in other systems. This is exciting because in science, when you scratch the surface, you often find that there is an entirely new world behind it.”
John Lueck, PhD, Assistant Professor, Pharmacology and Physiology, University of Rochester Medical Center
More Questions than Answers
Using the extra information about the Csx28’s structure via the use of high-resolution cryo-EM, the study group is starting to investigate the protein function. Many questions arise. Why is there a huge hole in the membrane if the goal is protection? The team discovered that Csx28 is not active when Cas13 is not around. What drives it to turn active in defense? How long does it stay active? What does it allow via the membrane?
Getting an insight into the underlying biochemistry behind the closing and opening of the pore will share knowledge on how CRISPR-Cas13 utilizes it as part of its defense and offer a jumping-off point for the examination of membrane proteins throughout other CRISPR systems.
This finding is unexpected and raises all kinds of new questions about how bacteria protect themselves and what they are doing to survive infection. It is also a very interesting interface between RNA biology, CRISPR, structural biology, and membrane biology. While there is no immediate medical relevance or application, the ideas that boil up from this could be very powerful.”
Mark Dumont, PhD, Professor, Biochemistry and Biophysics, University of Rochester Medical Center
Lueck said, “It is very rare for one study to have this many thought-provoking pieces that it brings several different fields together. And because the concepts are brand new, future work won’t be burdened by dogma. Any time people can bring fresh, unfettered ideas to the table it is really good for science.”
Source:
Journal reference:
Vanderwal, A. R., et al. (2023) Csx28 is a membrane pore that enhances CRISPR-Cas13b–dependent antiphage defense. Science. https://doi.org/10.1126/science.abm1184.