Reviewed by Danielle Ellis, B.Sc.Aug 8 2023
The mitochondria, sometimes known as the “powerhouses of the cell,” are widely recognized for their function as energy producers, but these organelles are also essential for preserving general health. Although mitochondrial stress is linked to aging and age-related diseases, such as neurodegeneration, the molecular processes behind this mitochondrial stress signaling are still poorly understood. A crucial stage in this process has now been discovered by a study conducted by Scripps Research scientists.
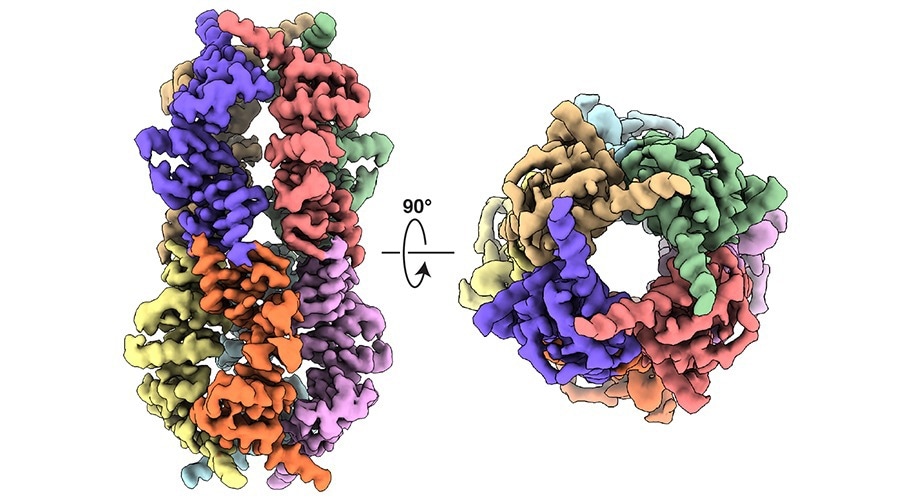 A structural depiction of a mitochondrial protein structure that initiates a cell-wide stress response. Using electron microscopy, Scripps Research scientists showed that this protein complex is made up of eight identical fragments of a protein called DELE1 that bind together into a highly symmetrical cylindrical. Image Credit: Jie Yang, Scripps Research
A structural depiction of a mitochondrial protein structure that initiates a cell-wide stress response. Using electron microscopy, Scripps Research scientists showed that this protein complex is made up of eight identical fragments of a protein called DELE1 that bind together into a highly symmetrical cylindrical. Image Credit: Jie Yang, Scripps Research
The new study, which was published on August 7th, 2023, in the journal Nature Structural & Molecular Biology, demonstrates how a mitochondrial protein structure is required to activate the cell's integrated stress response (ISR), a vital system that aids in cell health maintenance. In the future, age-related disorders could be treated by targeting this mitochondrial structure, which is composed of the protein DELE1.
Understanding the molecular details of this signaling pathway could help us potentially develop treatments for a range of diseases, such as neurodegenerative diseases, cancer and heart disease.”
Jie Yang, PhD, Study First Author and Postdoctoral Fellow, Scripps Research
Mitochondria must constantly perceive and react to stresses like viral infections and iron deficiencies to sustain cellular function and health. However, as people become older, they become less capable of doing so.
Just like every other part of our body, mitochondria age and become slightly less productive. When you have this loss of mitochondrial productivity, your cells don't have as much energy to fight different stressors, and many people believe that is a major trigger of neurodegeneration.”
Kelsey Baron, Study Co-Author and Graduate Student, Scripps Research
The ISR is one mechanism through which mitochondria respond to stress. Although prior studies have indicated that the DELE1 protein is crucial in triggering this integrated stress response, little is currently known about the protein’s molecular makeup. Understanding and treating diseases linked to mitochondrial stress need the structural characterization of DELE1.
The C terminus of DELE1, a fragment known to play a key role in the ISR’s initiation, was the focus of the study. The fact that this fragment was heavier than they had anticipated upon isolation showed that more than one copy of the protein fragment was tied together.
The scientists used electron microscopy to demonstrate that this protein complex (or oligomer) was an octamer, which is an extremely symmetrical cylinder made up of eight identical fragments.
It was completely unexpected that it was forming this much larger, oligomeric structure. It is kind of like two four-legged spiders whose legs are intertwined to form this flexible cylindrical structure.”
Gabriel Lander, PhD, Study Co-Senior Author and Professor, Department of Integrative Structural and Computational Biology, Scripps Research
More than 12,000 electron microscope images of the octamer were taken by the researchers, and after that, they employed algorithms to create a three-dimensional structural model. Then, by examining the locations of various amino acids (the components of proteins) inside the structure, scientists were able to determine which amino acids are responsible for binding and constructing the octamer.
The researchers next inserted mutations into a few of the essential amino acids, which would prevent DELE1 from binding together, to determine whether this oligomerization of DELE1 is necessary to activate the ISR. They were unable to activate the ISR in cultured cells that had this mutant, non-oligomerizable variant of DELE1, indicating that oligomerization is necessary for activating this stress signaling system.
The researchers next inserted mutations into a few of the essential amino acids, which would prevent DELE1 from binding together, to determine whether this oligomerization of DELE1 is necessary to activate the ISR. They were unable to activate the ISR in cultured cells that had this mutant, non-oligomerizable variant of DELE1, indicating that oligomerization is necessary for activating this stress signaling system.
The next step, according to the researchers, is to figure out how to control these pathways using this structural knowledge, especially in the context of various diseases and disorders.
Luke Wiseman, PhD, Study Co-Senior Author and a professor in the Department of Molecular medicine at Scripps Research, added, “Knowing that this oligomerization step is a potential site of regulation gives us a platform for potential drug development. We think that targeting this pathway has potential for improving outcomes in a variety of different disorders.”
Source:
Journal reference:
Yang, J., et al. (2023). DELE1 oligomerization promotes integrated stress response activation. Nature Structural & Molecular Biology. doi.org/10.1038/s41594-023-01061-0