In a recent release in Nature Communications, a collaborative research group comprising Johannes Gutenberg University Mainz (JGU), the University of Cologne, and the University of Oldenburg has disclosed their discoveries regarding the operation of a distinctive cryptochrome protein (Cry).
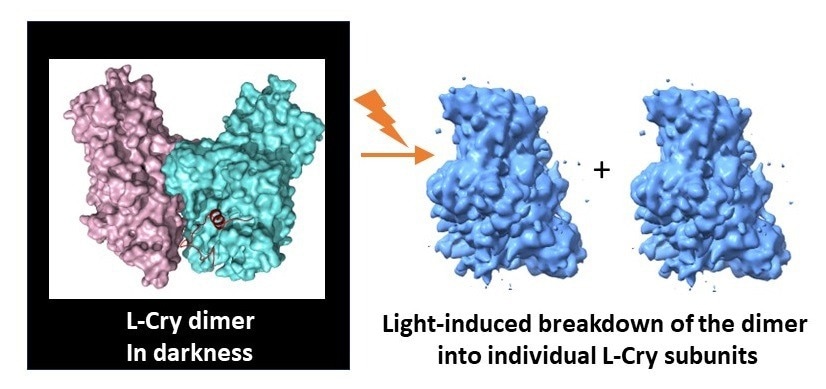 When exposed to bright illumination, the L-Cry protein dissociates into its two subunits. Image Credit: Eva Wolf and Hong Ha Vu.
When exposed to bright illumination, the L-Cry protein dissociates into its two subunits. Image Credit: Eva Wolf and Hong Ha Vu.
These proteins are present in various organisms, playing crucial roles in light-regulated biological processes. For instance, the marine bristle worm Platynereis dumerilii utilizes a specific Cry protein known as L-Cry to distinguish between sunlight and moonlight, as well as different moon phases.
This capability is vital for the worms to synchronize their reproduction with the full moon phase, guided by an internal monthly calendar referred to as a circalunar clock.
The research team in Cologne utilized their university’s cryo-electron microscopy platform to visualize the three-dimensional structure of the L-Cry protein under varying light conditions.
The results from structural analyses, coupled with biochemical investigations primarily conducted at Mainz University, unveiled that in the absence of light, L-Cry forms a unique dimer arrangement comprising two subunits connected by a stable link. Conversely, under intense sunlight-like illumination, it disassembles into its subunits or monomers.
What makes this discovery even more distinctive is not only the unusual spatial arrangement of the two subunits in the dark, which differs from other Cry proteins, but also the atypical direction of light-induced changes.
Typically, other Cry proteins undergo the reverse process, transitioning from monomer arrangements in the dark to dimer or higher oligomer arrangements in the light. The research team identified key structural features in the protein responsible for this unconventional behavior.
Moreover, the knowledge of the three-dimensional structure empowered the researchers to introduce targeted mutations in the L-Cry protein, allowing them to further characterize its role as a photoreceptor.
Our findings could explain how L-Cry manages to distinguish between sunlight and moonlight: Intense sunlight always activates both subunits of the dimer simultaneously, which initiates its breakdown into individual subunits. The significantly weaker moonlight, however, statistically only activates one of two subunits.”
Eva Wolf, Study Lead and Professor, JGU Institute of Molecular Physiology, Mainz University
The study’s findings underscore the distinctiveness of L-Cry compared to the highly varied Cry proteins, each with its diverse set of functions. Additionally, these proteins are believed to serve as sensor proteins, contributing to the perception of the Earth's magnetic field in birds.
First Steps of Decoding the Molecular Processes of the Circalunar Clock
“Working with light-sensitive proteins is always a challenge. When preparing the L-Cry proteins for analysis, we need to carry out all experimental processes in the dark or under specifically defined red light conditions to prevent unintentional pre-activation of these very light-sensitive proteins, stated Hong Ha Vu, a doctoral candidate in the JGU research group of Professor Eva Wolf and a major contributor to the study.
Vu added, “For the functional characterization of L-Cry, it is also necessary to use lighting conditions similar to underwater natural sunlight and moonlight illumination of the kind that the bristle worms encounter in their natural habitat. Only then we can compare the specific properties of L-Cry in its role as a sunlight and moonlight receptor with those of other cryptochromes.”
Our investigations have provided important new insights into how this most unusual sunlight and moonlight receptor works. Furthermore, our structural and molecular mechanistic insights into L-Cry's function have opened up future avenues of research that should help us better understand the still largely unknown molecular processes involved in synchronization of the circalunar clock with the moon phases.”
Eva Wolf, Study Lead and Professor, JGU Institute of Molecular Physiology, Mainz University
Source:
Journal reference:
Vu, H. H., et al. (2023) A marine cryptochrome with an inverse photo-oligomerization mechanism. Nature Communications. doi.org/10.1038/s41467-023-42708-2.