Recent findings from the lab of Jessica Thaxton, PhD, MsCR, in the UNC School of Medicine's Department of Cell Biology and Physiology, as well as associates in the Lineberger Comprehensive Cancer Center's Immunotherapy Group, have provided new insights into T-cell metabolism that may improve immunotherapies that depend on T cells to combat cancer.
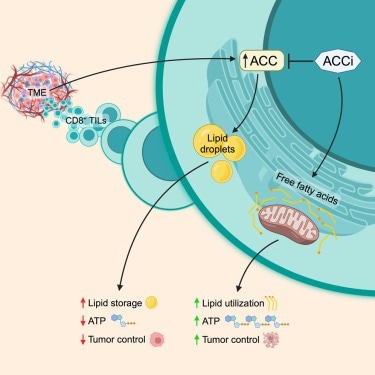 A graphical abstract showing how normal ACC expression causes lipid storage and inhibition of ACC leads to ATP production. Image Credit: Hunt et al.
A graphical abstract showing how normal ACC expression causes lipid storage and inhibition of ACC leads to ATP production. Image Credit: Hunt et al.
T cells are frequently referred to as “assassins” or “killers” due to their ability to plan and execute missions to locate cancer cells, viruses, and bacteria throughout the body. Despite their strength, T cells lose their ability to fight cancer once they enter the environment of a solid tumor. This has been demonstrated by recent research.
The goal of the study was to determine why T cells in tumors do not maintain energy. The team was led by Jessica Thaxton, Ph.D., MsCR, Associate Professor of cell biology and physiology and co-leader of the UNC Lineberger Comprehensive Cancer Center’s Cancer Cell Biology Program.
The Thaxton Lab, led by Katie Hurst, MPH, and fourth-year graduate student Ellie Hunt, used their knowledge of tumor immunity and metabolism to discover that T cells store fat instead of burning it for energy due to a metabolic enzyme called acetyl-CoA carboxylase (ACC).
Our discovery fills a long-standing gap in knowledge regarding why T cells in solid tumors don’t appropriately generate energy. We inhibited the expression of ACC in mouse cancer models, and we observed that T cells were able to persist much better in solid tumors.”
Jessica Thaxton, Department of Cell Biology and Physiology, University of North Carolina School of Medicine
The new research and immunotherapeutic approaches, which were published in Cell Metabolism, may help patients receive more benefit from a variety of T-cell therapies, including checkpoint and chimeric antigen receptor (CAR) T-cell therapies.
It has long been known in the field of cancer immunotherapy that T cells inside solid tumors are unable to produce adenosine triphosphate, or ATP, the cellular energy.
The year 2019 saw research in Thaxton's lab on a T cell that exhibited peak antitumor activity. Hurst and Thaxton employed a proteomics screen in a paper published in Cancer Immunology Research to find the enzymes connected to these T cells' ideal antitumor metabolism. The two found via this screen that T cell ATP production in tumors may be inhibited by ACC expression.
A crucial molecule called ACC, which is involved in numerous metabolic processes, prevents cells from metabolizing fat and utilizing it as energy in the mitochondria.
Acetyl-CoA carboxylase can drive the balance between storing lipids versus breaking down those lipids and feeding them into the citric acid cycle for energy. If ACC is flipped ‘on’, cells generally store lipid. If ACC is ‘off’, cells tend to use the lipid in their mitochondria to make ATP.”
Jessica Thaxton, Department of Cell Biology and Physiology, University of North Carolina School of Medicine
Hunt's proficiency with confocal imaging allowed the research team to see lipid reserves in T cells separated from various cancer types. The team's theory that T cells were storing lipids rather than breaking them down was supported by this observation and additional tests.
Thaxton's group then experimented with “deleting” ACC from the image using CRISPR Cas9-mediated gene deletion. The quantity of lipid storage in T cells decreased quickly, and the researchers saw fat moving to the mitochondria to be converted into energy.
According to Thaxton's current theory, T cells may require a “delicate balance” of lipids to survive in solid tumors, with low levels of fats being kept in reserves and a specific amount of lipids devoted to killing cancer cells.
The most recent research may help to improve treatments using chimeric antigen receptor (CAR) T cells. With the use of this state-of-the-art technology, cancer patients’ T cells are extracted, altered in a lab to find tumor cells, and then reinfused to battle the patient’s cancer. Thaxton’s lab has produced some preliminary data showing that excess lipid stores are present even in the manufactured T cells.
In an effort to eliminate the need to remove and reintroduce cells into the body, the lab is beginning to examine patient samples in an attempt to determine how researchers might be able to directly flip the ACC metabolic switch in patient tumors. However, more research is needed to ascertain how this might impact other immune cell populations in the body, like macrophages.
Source:
Journal reference:
Hunt, G., E., et al. (2024) Acetyl-CoA carboxylase obstructs CD8+ T cell lipid utilization in the tumor microenvironment. Cell Metabolism. doi.org/10.1016/j.cmet.2024.02.009