Swedish researchers have enhanced a method for transforming ordinary skin cells into neural stem cells, a development they believe narrows the accessibility gap for personalized cell-based therapies for Alzheimer's and Parkinson's diseases.
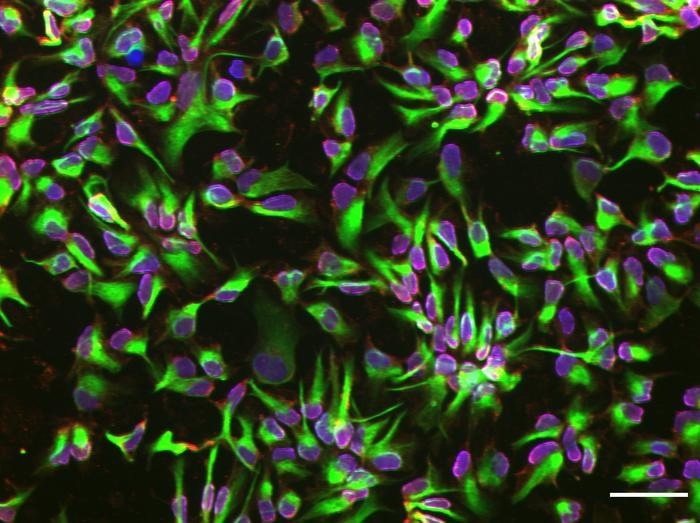 Neural stem cells differentiated with the chip platform. Image Credit: Saumey Jain, Dimitrios Voulgaris, Surangrat Thongkorn, Rick Hesen, Alice Hägg, Mohsen Moslem, Anna Falk, Anna Herland/KTH Royal Institute of Technology
Neural stem cells differentiated with the chip platform. Image Credit: Saumey Jain, Dimitrios Voulgaris, Surangrat Thongkorn, Rick Hesen, Alice Hägg, Mohsen Moslem, Anna Falk, Anna Herland/KTH Royal Institute of Technology
The research team has created a novel and quicker method for reprogramming human skin cells into Induced Pluripotent Stem Cells (iPSCs) and then further converting them into neural stem cells by using a specially designed microfluidic device.
According to Saumey Jain, the study's first author, the platform could enhance and reduce the cost of cell therapy by making it simpler for a patient's body to match and accept the cells. KTH Royal Institute of Technology researchers published their findings in the journal Advanced Science.
According to the study's senior author, Anna Herland, it showed how microfluidics could be utilized for the first time to steer iPSCs toward becoming neural stem cells.
It takes two steps to engineer the conversion of normal cells into neural stem cells. The cells are induced into pluripotent stem cells (iPSCs), which can differentiate into various cell types, through a procedure that involves exposing the cells to biochemical cues.
After that, they are placed in a culture medium that replicates the developmental processes and signaling cues involved in the formation of the nervous system. Neural differentiation is the stage at which cells decide to become neural stem cells.
For the past nine years or so, microfluidic devices have replaced well plates as the medium for this type of laboratory work. According to Herland, the new platform is an advancement in microfluidics for the production of iPSCs and the differentiation of neural stem cells.
They discovered that, in comparison to cells differentiated in a traditional well plate format, the microfluidic platform allowed a boosted commitment to their neural fate at an earlier point using cells from a human skin biopsy.
We documented that the confined environment of a microfluidic platform boosts neural stem cell generation commitment.”
Anna Herland, Study Senior Author, KTH Royal Institute of Technology
According to Jain, the microfluidic chip can be easily made with PolyDiMethyl Siloxane (PDMS) and can save a significant amount of money on reagents and cellular input because of its microscale size.
According to him, the platform is easily modifiable to allow for adaptability for differentiation into different cell types. It can be automated, offering a closed system that guarantees dependability and consistency in the generation of remarkably uniform cell populations.
Jain said, “This marks a step towards making personalized cell-based therapies for Alzheimer's and Parkinson's accessible.”
Source:
Journal reference:
Jain, S., et al. (2024) On-Chip Neural Induction Boosts Neural Stem Cell Commitment: Toward a Pipeline for iPSC-Based Therapies. Advanced Science. doi.org/10.1002/advs.202401859