Reviewed by Lexie CornerMay 8 2025
Scientists at Institut Curie, CNRS, and Inserm have developed a new class of molecules that target and eliminate cancer cells resistant to conventional therapies - cells often responsible for relapse and metastasis. This breakthrough is based on identifying the specific cellular location where ferroptosis begins, a form of iron-dependent cell death driven by oxidative damage to cell membranes.
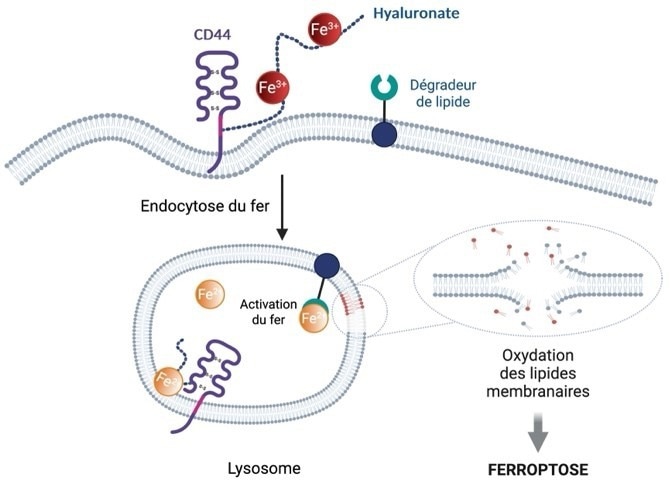 Ferroptosis diagram. Iron is internalized in the cell via the protein CD44 present on its surface, allowing it to acquire metastatic properties and tolerance to standard treatments through epigenetic reprogramming, which plays a key role in cell adaptation. The activation of lysosomal iron by a phospholipid degrader causes the oxidation and rupture of cell membranes, leading to cell death. Image Credit: Centre national de la recherche scientifique (CNRS)
Ferroptosis diagram. Iron is internalized in the cell via the protein CD44 present on its surface, allowing it to acquire metastatic properties and tolerance to standard treatments through epigenetic reprogramming, which plays a key role in cell adaptation. The activation of lysosomal iron by a phospholipid degrader causes the oxidation and rupture of cell membranes, leading to cell death. Image Credit: Centre national de la recherche scientifique (CNRS)
Current anticancer treatments primarily target rapidly multiplying primary tumor cells but do not effectively eliminate certain cancer cells that can adapt to treatment and possess high metastatic potential. However, metastases account for 70 % of cancer-related deaths.
A French research team from Institut Curie, the CNRS, and Inserm has recently developed a new class of small molecules that induce cell membrane destruction, leading to cell death. The study, led by scientists at the Laboratory of Biomedicine (Institut Curie/CNRS/Inserm), focuses on the unique characteristics of drug-tolerant persister cancer cells, which have a strong metastatic potential.
These cells exhibit elevated levels of the protein CD44 on their surface, enabling them to absorb more iron. This makes them more aggressive and resistant to conventional treatments. As a result, they are more susceptible to ferroptosis, a form of cell death triggered by iron-induced oxidation and membrane lipid degradation.
Researchers led by Raphaël Rodriguez discovered that iron within lysosomes can initiate cell death and influence the formation of internal membrane compartments. In the lysosomal environment, iron can react with hydrogen peroxide to produce oxygen-centered radicals - highly reactive chemical species that damage cell membranes.
This damage propagates throughout the cell, generating lipid peroxides in the membranes of various organelles and ultimately inducing cell death. Ferroptosis arises from the cell's inability to repair this membrane damage.
Based on these findings, the researchers designed and synthesized a novel class of small molecules - phospholipid degraders - that trigger ferroptosis. These molecules include one component that targets the plasma membrane and enables accumulation in lysosomes via endocytosis, and another that binds iron and enhances its reactivity. This mechanism exploits the iron-rich lysosomal compartments of pro-metastatic cancer cells to initiate ferroptosis.
Fentomycin (Fento-1) was engineered to be fluorescent, allowing researchers to visualize its localization in lysosomes using high-resolution microscopy.
Following Fento-1 administration, the researchers observed a significant reduction in tumor growth in preclinical models of metastatic breast cancer. They also noted a strong cytotoxic effect on biopsies from patients with pancreatic cancer and sarcoma, cancers for which standard chemotherapy is often ineffective, demonstrating the treatment’s efficacy at the preclinical level.
Clinical trials are needed to determine whether this ferroptosis-inducing approach could complement existing chemotherapy, particularly by targeting pro-metastatic, treatment-resistant cancer cells.
Source:
Journal reference:
Cañeque, T., et al. (2025) Activation of lysosomal iron triggers ferroptosis in cancer. Nature. doi.org/10.1038/s41586-025-08974-4.