Reviewed by Lexie CornerJun 11 2025
Microglia are specialized immune cells that make up around 10 % of all cells in the brain and spinal cord. They work by removing pathogenic bacteria, dead cells, aggregated proteins, and soluble antigens that might damage the brain. During development, they also assist in building neural circuits that enable certain brain functions.
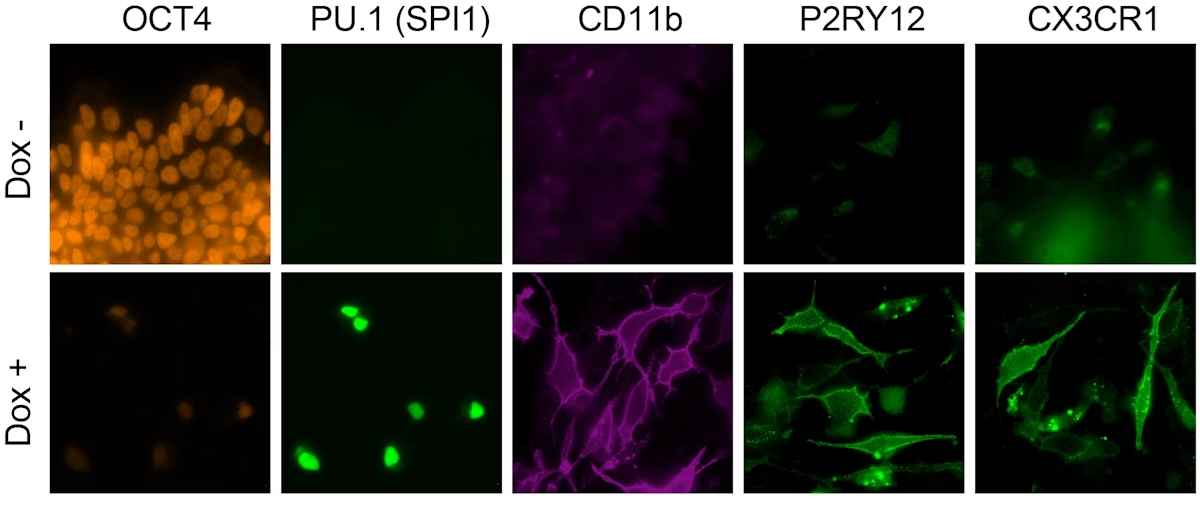 This image shows how human iPSCs in which the microglia-specifying six-TF cocktail is switched on (lower row), in contrast to iPSCs in which they remain switched off (upper row), abandon their pluripotency stage (marked by the protein OCT4) and adopt the fate of microglia cells (marked by the proteins CD11b, P2RY12, and CX3CR1). Image Credit: Wyss Institute at Harvard University
This image shows how human iPSCs in which the microglia-specifying six-TF cocktail is switched on (lower row), in contrast to iPSCs in which they remain switched off (upper row), abandon their pluripotency stage (marked by the protein OCT4) and adopt the fate of microglia cells (marked by the proteins CD11b, P2RY12, and CX3CR1). Image Credit: Wyss Institute at Harvard University
When microglia do not function properly, they may contribute to neuroinflammation and fail to clear damaged cells or toxic protein clusters. These include neurofibrillary tangles and amyloid plaques, which are characteristic of Alzheimer’s disease.
This dysfunction is linked to several neurodegenerative diseases, such as Alzheimer’s, Parkinson’s, Huntington’s disease, Amyotrophic Lateral Sclerosis (ALS), and multiple sclerosis. In some cases, neuroinflammation occurs before harmful protein aggregates form, potentially increasing the extent of aggregation.
Studying microglia remains a challenge. Human microglia can only be obtained through biopsies, and rodent microglia differ significantly from human ones in key biological aspects.
Due to limited access to human microglia, researchers have developed methods to produce them from stem cells in the lab. However, these processes are slow and costly, often taking weeks to generate usable cells.
A team at the Wyss Institute, led by George Church, Ph.D., a core faculty member at Harvard University and Harvard Medical School, has developed a faster method. They can now produce microglia from induced pluripotent stem cells (iPSCs) in four days. Traditional methods take around 35 days and often produce less functionally accurate cells.
Their approach builds on a previous platform called “TFome™,” which uses transcription factors (TFs) to guide stem cells through various stages of differentiation more efficiently than other methods. TFs are proteins that control gene expression and influence cell development.
In this study, the researchers created libraries of microglia-specific TFs. They tested various combinations to identify those that best converted iPSCs into microglia-like cells.
To assess the quality of the resulting cells, the team used single-cell RNA sequencing (scRNA-seq). This allowed them to compare gene expression patterns with those of real human microglia. Through this analysis, they identified a set of six transcription factors that reliably and quickly generate microglia-like cells.
Their findings were published in Nature Communications.
We advance transcription factor libraries as a platform technology here in a synthetic biology paradigm. By integrating it with single-cell RNA data-driven analysis and iterative rounds of optimization, we succeeded in creating much sought-after human microglia cells in the dish.
George Church, Founding Member, Wyss Institute for Biologically Inspired Engineering, Harvard University
“This cell differentiation approach can open many avenues of brain disease-focused research and new therapeutic perspectives. Equally relevant, it can be applied to the generation of other hard-to-get and therapeutically relevant cell types that require complex transcriptional scenarios.”
Organoid Beginnings
In 2021, co-authors Alex Ng, Ph.D., and Parastoo Khoshakhlagh, Ph.D., identified individual TFs that could generate specific cell types. These TFs were intended for use in faster manufacturing of advanced cell therapies. They also created a library of 1,732 human TFs and their variants, which became a core part of the TFome™ platform.
To commercialize this cell engineering method and develop new cell therapy products, Ng, Khoshakhlagh, Church, and former HMS researcher Cory Smith, Ph.D., founded GC Therapeutics. In the current study, Church’s team at the Wyss Institute and Harvard Medical School expanded the TFome™ platform’s application.
The team became interested in microglia after earlier research on lab-grown brain organoids - three-dimensional tissues that mimic some functions and structure of the brain.
In our efforts to develop human brain organoids with features from patients who suffer from specific brain disorders, we were able to create tissue constructs containing iPSC-derived neuronal cells, oligodendrocytes, stromal and vascular cells, using TFome™ technology, but to capture aspects of neuroinflammation in our studies, we also needed to be able to incorporate microglia.
Jenny Tam, Ph.D., Study Co-Corresponding Author and Director, Wyss Institute for Biologically Inspired Engineering, Harvard University
Church, Tam, co-author Katharina Meyer, Ph.D., and others at Wyss are now applying brain organoids and the TFome™ platform in their CircaVent drug discovery system. This work focuses on potential treatments for mental health disorders such as bipolar disorder.
“However, we knew that generating microglia would likely require a complex combination of TFs,” Tam added.
To create human microglia cells in vitro, using the TFome™ process, we realized that we do not have to screen the entire library, but, based on a large body of earlier developmental and disease studies, could start by making a smart choice. So, we came up with a collection of 40 TFs whose induced gene expression profiles were typical for primary human microglia and devised a strategy to express random combinations of five to seven of them in single iPSCs.”
Songlei Liu, Ph.D., Study First Author and Graduate Student, Wyss Institute for Biologically Inspired Engineering, Harvard University
Liu led the microglia study in Church's group and now works as a platform technology scientist at nChroma Bio.
To identify which TF combinations best triggered microglia gene expression, the team collaborated with Soumya Raychaudhuri, M.D., Ph.D., a professor at Brigham and Women's Hospital and Harvard Medical School, and a co-corresponding author on the study.
Raychaudhuri, along with his former postdoctoral fellow Fan Zhang, Ph.D., and Li Li, Ph.D., a postdoctoral fellow in Church’s group, applied statistical and computational tools to analyze scRNA-seq data. They examined gene expression in thousands of individual cells after a few days of culture and ranked the TF combinations based on how closely the cells resembled microglia.
Zhang, now an Assistant Professor at the University of Colorado, and Li are joint first authors of the study. The initial screening identified three TFs (SPI1, CEBPA, and FLI1) that together activated a microglia-specific differentiation pathway in iPSCs.
Next Round is on the Platform
Although the cells showed the expected microglia-like gene expression and shape, the researchers found that they had not yet reached the functional maturity of primary human microglia.
“We argued that we could go through iterative rounds of this design-screen-validate cycle, meaning that adding new TFs in consecutive rounds could improve outcomes and lead to more superior TF combinations,” Liu noted.
To improve the results, the researchers identified 42 additional transcription factors. These were selected by comparing gene expression in iPSCs treated with the initial three-TF cocktail to that of primary human microglia. They looked for TFs that remained underexpressed in the iPSCs and used computational tools to guide their selection.
Their analysis identified three more TFs - MEF2C, CEBPB, and IRF8 - that, when added to the mix, further improved microglia differentiation. This expanded the TF cocktail to six factors.
Liu and his team then tested whether the resulting cells responded to disease-relevant signals. When exposed to interferon gamma (IFNg), a cytokine that increases during infection, the cells showed gene expression patterns similar to activated human microglia.
They also treated the cells with TDP-43, a protein that accumulates in ALS. This altered gene expression in ways consistent with responses seen in primary microglia.
“Building on this proof-of-concept study, we believe that by identifying additional TFs, developing methods to further fine-tune the expression strength of individual TFs, and optimizing their order of appearance within the four-day timeframe, we can refine specific microglia identities and potentially create microglia subtypes with specialized functions in the brain,” Liu concluded.
Source:
Journal reference:
Liu, S., et al. (2025) Iterative transcription factor screening enables rapid generation of microglia-like cells from human iPSC. Nature Communications. doi.org/10.1038/s41467-025-59596-3.