Reviewed by Lauren HardakerSep 5 2025
Professor Zhang, a chemical and biomolecular engineering professor at UC Berkeley, is working to distinguish between the healthy and unhealthy bacteria in the human mouth. She aims to promote a probiotic oral microbiome by increasing the population of beneficial bacteria.
 Image credit: sheff/Shutterstock.com
Image credit: sheff/Shutterstock.com
The human oral microbiome contains hundreds of bacterial species, many of which aggregate on teeth to form plaque. Earlier research investigated which species contribute to cavities by producing acids that erode enamel. However, scientists have discovered that individual species are not uniformly beneficial or harmful; each species can consist of hundreds of distinct strains, which vary in their potential to promote cavities.
Rather than examining individual species or strains, Zhang and her team analyzed the DNA of all oral bacteria, the metagenome, looking for gene clusters linked to cavities.
In a study published on August 19 in Proceedings of the National Academy of Sciences, they identified a gene cluster that produces two molecules that enable the oral bacterial community, both beneficial and harmful, to adhere to teeth and form a robust biofilm.
The researchers identified this gene cluster in particular, but not all, strains of several well-known cavity-causing bacteria, including Streptococcus mutans, the primary contributor to tooth decay. Zhang envisions introducing this gene cluster into beneficial bacteria to enhance their adherence to teeth, thereby displacing the acid-producing species that promote cavities.
Particular strains belonging to the same species can be a pathogen or a commensal or even probiotic. After we better understand these molecules’ activity and how they can promote strong biofilm formation, we can introduce them to the good bacteria so that the good bacteria can now form strong biofilms and outcompete all the bad ones.
Wenjun Zhang, Professor, Chemical and Biomolecular Engineering, UC Berkeley
This research was funded by the National Institute of Dental & Craniofacial Research, part of the National Institutes of Health (R01DE032732).
“Specialized” Metabolism
The gene cluster was discovered by analyzing an online database of metagenomic sequences from the oral microbial communities of human volunteers. Berkeley graduate student McKenna Yao led a statistical analysis to identify clusters associated with oral disease. Yao then cultivated the bacteria to analyze and pinpoint the metabolites produced by these clusters.
The metabolites the gene cluster produces are small molecules composed of peptides and fatty acids. One molecule works like a "glue" to clump cells, while the other acts like a "string" to form chains. Together, they allow bacteria to build communities - the sticky substance on the teeth, instead of floating as individual cells.
This newly discovered gene cluster contains about 15 DNA segments that form a self-contained metabolic pathway. While not essential for the bacteria's survival, this pathway significantly impacts their environment, such as the teeth. Zhang refers to this as a "specialized" metabolic network, a term she prefers over "secondary," noting that similar networks in soil bacteria have proven to be a fertile source for antibiotics.
These specialized metabolites enhance survival in certain ways. Many, for example, are antibiotics, so they can kill other bugs, or others are involved in metal acquisition — they help the bacteria monopolize the resources in their environmental niche. Being able to produce these, especially in a microbial community, helps the bacteria boot out the other guy and guard their resources.
McKenna Yao, Graduate Student and Study First Author, UC Berkeley
The role of specialized metabolic networks and secondary metabolites in the human microbiome has largely remained unstudied. However, Zhang's team previously discovered a gene cluster in oral bacteria that produces a new antibiotic and another cluster that creates sticky molecules for biofilm formation.
The newly reported gene cluster demonstrates the importance of these metabolites in human health. Understanding these sticky molecules, now dubbed mutanoclumpins, could play a significant role in reducing cavities.
“We are looking for something which is correlated with cavities, with disease. If one day we can prove that, under certain conditions, this is really a bad molecule you want to prevent, we might develop genetic or chemical inhibitors to inhibit their production, so hopefully the bacteria will not make them, and you have fewer cavities. Meanwhile, we also look at other molecules correlated with health, allowing a simple strategy to directly engineer the microbes to make more of them,” said Zhang.
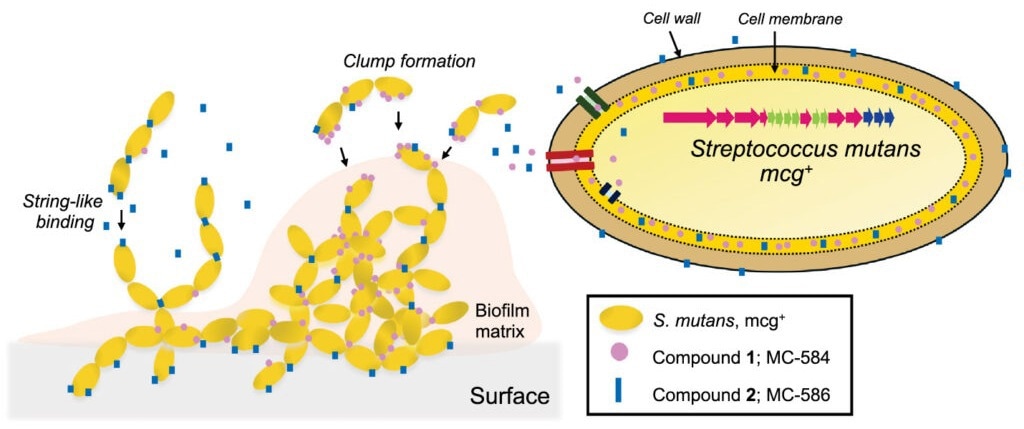
Binding phenotype and localization of mutanoclumpins. (A) SEM images of planktonic Smu102, ΔmcgB, and chemically complemented planktonic ΔmcgB with 1, 2, and both, imaged at various magnifications for image clarity with a current of 93.3pA and a voltage of 10.0 kV. (Scale bars are 5 μm.) (B) Extraction of 1 and 2 from subcellular fractionation of ΔmcgB with 1 and 2 readded independently (Top) and natural producer Smu102 (Bottom). 2 μg/mL of 1 or 2 was added to ΔmcgB in separate experiments. 1 and 2 were extracted simultaneously from Smu102. Error bars represent SD of three biological replicates.
One species of bacteria that could benefit from a boost is Streptococcus salivarius, which is currently marketed as an oral probiotic. While it appears to promote oral health, it does not form a strong biofilm that sticks to teeth and instead dissipates quickly. Zhang suggests adding strong biofilm-forming molecules to S. salivarius to see if it can function more effectively as a probiotic.
“Our future work will be to create a broad map of the collection of these specialized metabolites to look at collectively what this dynamic, complex community on your teeth is making,” said Zhang.
Yao noted, however, that “the best way you can remove the biofilm on your teeth is to brush. We believe that there’s actually a better way of disrupting that biofilm, but we’re just beginning to understand what the complexity is within the mouth.”
Source:
Journal reference:
Zhang, W., et al. (2025) Synergistic action of specialized metabolites from divergent biosynthesis in the human oral microbiome. Proceedings of the National Academy of Sciences. doi.org/10.1073/pnas.2504492122