This article discusses the implementation of an automated, high-density sample storage and sorting system at the MRC Epidemiology Unit, University of Cambridge. The unit is run by the Head of the Laboratory, Dr. Debora Lucarelli.
Automation has been key to the unit, enhancing the efficiency of their large-scale sample management and processing, the integrity of samples, as well as the speed and scope of their work.
Background
Biorepositories, biobanks, and other bio archives or collections store vast numbers of biological samples such as DNA. They provide a crucial resource for groups conducting biomedical research, including epidemiological studies i.e. researching the underlying factors of human disease.
These facilities curate and supply extensive collections of individual samples that have been gathered over time from participants of studies. For any epidemiological or other biomedical research study to be successful, it must include sufficient numbers to deliver statistically significant results.
The labor, time, cost to source, and sample numbers needed for analysis are substantial and frequently beyond most research groups' scope.
Volunteer samples already collected in biorepositories are advantageous to researchers, and their value for facilitating research on statistically significant populations that would otherwise not be feasible is increasingly being acknowledged.
Maintaining the integrity of stored samples in any collection is vital, considering factors that may impact long-term sample quality in how they are collected, handled, and ultimately stored.
Biological variation in samples, including those that experience hemolysis during sampling, or natural physiological variability in fat or glucose, may be minimized before being stored using careful collection and processing but never removed entirely.
Hemolysis can be reduced in blood samples using standardized sampling methods, rapid processing from whole blood to plasma/serum, rapid aliquoting, and freezing.
The sampling method and time to storage are critical. Storing large amounts of samples delivers numerous significant challenges, including tracking and sorting samples, finding sufficient space and minimizing the footprint, and maintaining samples at the proper protective temperatures (usually frozen or refrigerated).
This is a complex process operationally. Typically, samples are processed by numerous operators, which involves training, SOPs, and replicate equipment. Samples must be carefully logged (preferably LIMS) and transferred to a final repository. Individual samples will be utilized often over a prolonged period and must not be compromised.
Conventionally, tubes have been stored in boxes of samples requiring manual retrieval and numerous cycles of freezing and thawing. Large-scale sample management requires all curation and processing to be as efficient as possible.
Novel Automated Sample Storage and Cold Sorting Leads to Maximized Efficiency and Sample Integrity
Full or partial incorporation of high-density, automated tube storage, and temperature-controlled handling may resolve many problems and enhance the capacity of storage facilities of any size. Transporting samples to individual 2D barcoded tubes removes sample loss, cross-contamination, and unnecessary bulk retrieval and freeze/thawing.
Unique 2D barcodes for each sample allow complete LIMS software tracking at each stage of movement of the sample, in addition to providing thorough annotation of its history and analysis. Automating storage and retrieval speeds up the preparation of custom sample sets, substantially decreasing labor and manual handling.
Logging sample data facilitates the cherry-picking of sample sets based on multiple criteria, including sample type, age, or blood chemistry.
The arktic®, manufactured by SPT Labtech, is an automated high-density -80 °C sample storage and retrieval system. The arktic securely stores 2D barcoded tubes in a -80 °C environment that is safe and frost-free and utilizes innovative pneumatic technology to move storage tubes, reducing the use of moving parts for greater reliability.
A range of monitoring and backup systems ensures sample integrity, such as a 100% refrigeration backup system that automatically switches on if needed. arktic can handle various 2D barcode tubes in the SBS standard 96-sample rack format.
The system also enables quick cherry-picking of samples, only supplying the requested samples, meaning the unnecessary picking of non-required “innocent” samples is prevented. A full rack containing 96 samples may be delivered in only 10 minutes.
arktic allows as many as 140,000 2D barcoded tubes to be stored on a footprint smaller than 1.1m2. The pneumatic transport system utilized in arktic has a proven record for assured operation and reliability. This was introduced in 2001, in the first generation of comPOUND® storage systems by SPT Labtech.
The comPOUND is offered in RT, +4 °C, and -20 °C configurations and is compatible with a range of 2D barcoded tubes from various manufacturers. The comPOUND can access any sample in only 6 seconds and cherry-pick a full rack of 96 storage tubes within 10 minutes.
The comPOUND offers storage of up to 100,000 full-height storage tubes (such as 1.4 ml 2D barcoded tubes) or 200,000 half-height storage tubes (such as 0.5 ml 2D barcoded tubes).
The arktic and comPOUND are designed as scalable modular units to enable customers to address their current storage requirements with compact solutions, adding more units as their collections expand.
Various complimentary automation solutions provide the capacity for storing and retrieving sample tubes from multiple stores to a single point by employing SPT Labtech’s lab2lab technology.
This pneumatic delivery system enables much flexibility in the location of storage units, with the option of positioning multiple comPOUND and arktic systems across a site with delivery and collection to a single location (or even multiple locations). The arktic and comPOUND systems are straightforward to integrate with in-house and commercial LIMS systems.

Image Credit: SPT Labtech

 arktic (top), and comPOUND (bottom). Image Credit: SPT Labtech
arktic (top), and comPOUND (bottom). Image Credit: SPT Labtech
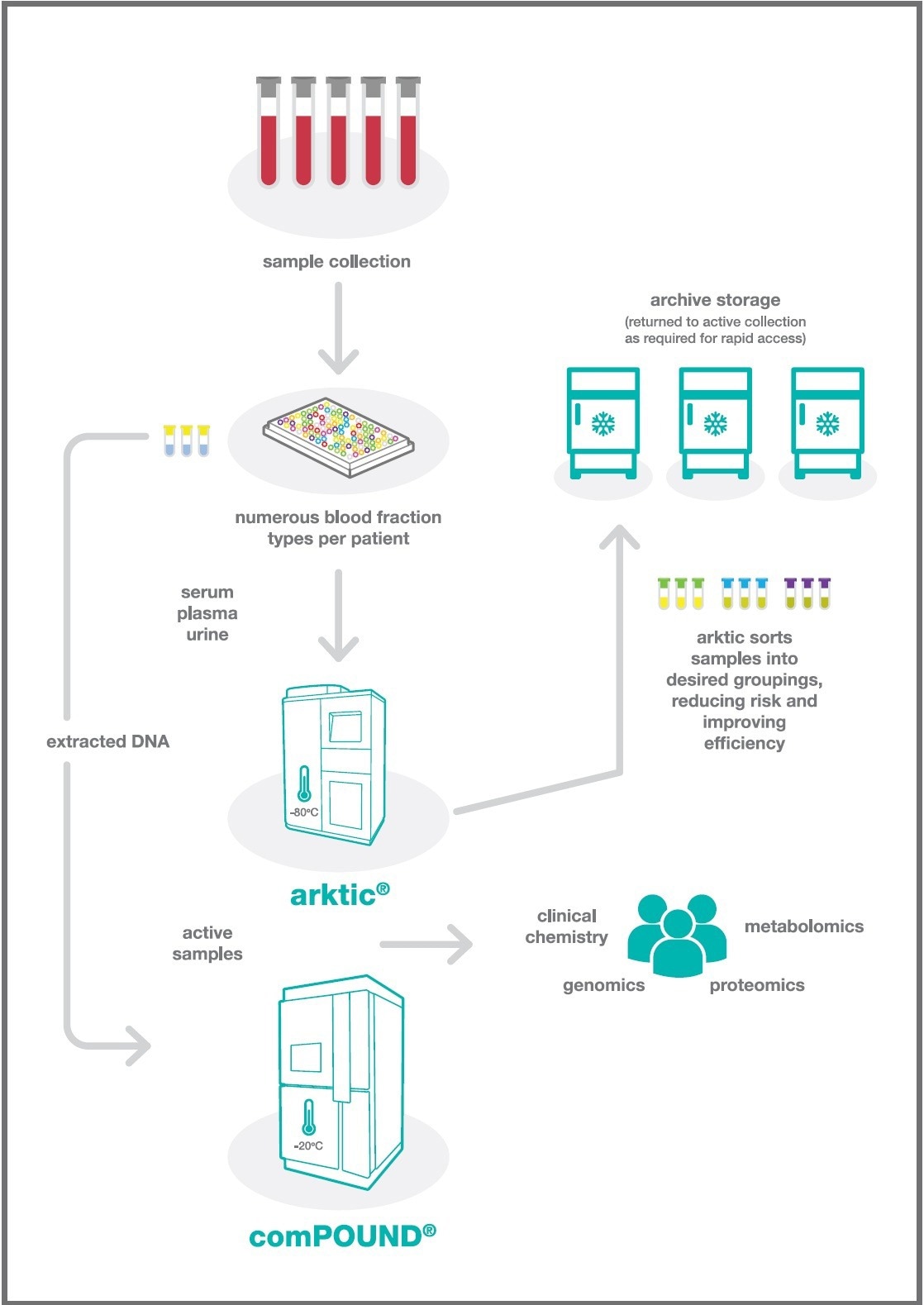
Figure 1. Workflow illustrating how a combination of arktic and comPOUND storage modules can be combined with existing large-scale manual storage in biorepositories and biobanks to facilitate sample management / organisation and arrangement / allocation /distribution / short term holding. Image Credit: SPT Labtech
Case Study: Innovative Clinical Research Sample Management at MRC Cambridge Addenbrookes
Epidemiological studies into obesity, type II diabetes, and other metabolic disorders are conducted at the MRC Epidemiology Unit. With samples being collected from numerous studies since 1990, the unit currently stores more than 1.5 million samples at 4 °C, -20 °C, -80 °C and -196 °C.
The unit collects whole blood, serum, plasma, PBMC, and urine samples from the participants of the studies. The extraction of DNA from whole blood is performed, with the resultant DNA being stored in the SPT Labtech comPOUND at 4 °C.
The remaining samples are divided and stored in 2D barcoded tubes. Racks that contain these tubes are subsequently placed in the SPT Labtech arktic for storage and re-arraying.
The laboratory holds and organizes samples for medium to large-scale studies (up to 40,000 samples in a study), typically case control-based or prospective cohort studies. The samples are normally irreplaceable and finite, so the unit's maintenance of sample integrity is the utmost priority.
The analysis of samples includes traditional clinical biochemistry, genetic analysis e.g. GWAS, nutritional biomarkers, various protein markers, and methylation-based analysis.
Sample management supports all the measurement activities at the unit. The goal is to achieve reliable, consistent, and reproducible data from all samples.
The MRC Epidemiology Unit has installed an automated system comprised of two arktic modules (60,000 tubes in one arktic and eventually 100,000 in the other) and a comPOUND to manage the storage and retrieval of more than 100,000 DNA samples. The novel application of the arktic for cold-sorting of samples is particularly interesting.
Methods and Workflow
Samples must be prepared within a standard timeframe in the clinics and laboratories, preferably within two hours from obtaining the sample from a participant to reaching cold storage. Samples are transported from field collection tubes to 2D-barcoded 1 mL tubes.
Subsequently, the arktic is employed to organize and reshuffle the samples within the store, keeping one aliquot of each sample type and freeing the remaining samples for storage in the archive manual freezers. Once stored, the arktic is also utilized for preparing orders overnight, involving the loading and dispensing a rack in a predefined arrangement.
The following morning, the samples are stamped out for specific assays utilizing more automated platforms.
The arktic provides agility to the measurement pipelines, enabling samples to be retrieved and organized within a few hours. This helps to prevent trips to a storage facility for the retrieval of samples, as well as avoiding the time involved in arraying samples on racks or plates, whilst facilitating the continuous tracking of samples.
EPIC-Norfolk Study – Automated Storage and Processing of Extracted DNA
The EPIC-Norfolk study presents a strong example of how automated processing has enabled a significant difference in sample quality. The EPIC-Norfolk study is a large study utilizing 25,000 DNA samples collected since 1993 from participants aged between 40 and 79 years.
Earlier, individual samples were condensed into 96-well microtiter plates and sealed. For such a sizable collection, retrieving individual samples was extremely labor intensive. There were major problems regarding sample loss, cross-contamination, and the numerous hours required to manually access and process the DNA samples.
After the introduction of the comPOUND, samples were transported and stored in individual 2D-barcoded tubes. This facilitated the automation of quantification, normalization, and storage, in addition to providing the capacity to maintain both a standard dilution working stock at 4 °C and a high concentration archive stock at -80 °C within the automated system.
This means the unit can rapidly select individual samples and prepare genetic analysis studies. This process would have formerly required months, but now only requires days. This has allowed scientists to focus on analyzing data, instead of wasting valuable time on the labor-intensive and error-prone job of sample picking.
Barcode scanning and LIMS software allow data to be captured at different points during laboratory processing and analysis, including sample concentration, volume, and location. This delivers a comprehensive audit trail for each sample, as well as more confidence in the quality of the analysis.
Conclusion
The arktic and comPOUND from SPT Labtech can be easily set up in biorepositories of any size to increase the efficiency, capacity, and quality of sample management. These high-density, automated storage solutions uphold sample integrity and deliver fast sample retrieval and organization of numerous samples in the minimum possible footprint.
The main advantages of employing these instruments are as follows:
arktic:
- High-density storage with a compact footprint of 140,000 tubes in just 1.1 m2
- Removes unnecessary bulk sample freeze/thaw with the use of automated, single tube selection and retrieval
- Retrieval of individual tubes from -80 °C storage in just 15 seconds or custom 96-vial rack combinations within 10 minutes
comPOUND:
- Provides storage of 100,000 x 1.4 mL or 200,000 x 0.5 mL vials (or a combination) at -20 °C, 4 °C or ambient temperature
- Enables the user to cherry-pick any sample within 10 seconds
- Provides straightforward, reliable, and proven pneumatic technology
- Easy to integrate with downstream applications
The arktic has revolutionized our sample collections. We are now able to track and reorganize samples automatically thus reducing a lot of the manual procedures previously involved in the process.”
Dr. Debora Lucarelli, Head of Laboratory, MRC Epidemiology Unit, University of Cambridge, UK
Sample integrity is the highest priority from sample collection from the patient and storage through to sample analysis and generation of data.”
Matt Sims, Head of Research Operations & Head of NIHR BRC-MRC BioRepository. MRC Epidemiology Unit, University of Cambridge School of Clinical Medicine
About SPT Labtech
We Design and Manufacture Robust, Reliable and Easy-to-Use Solutions for Life Science
We enable life scientists through collaboration, deep application knowledge, and leading engineering to accelerate research and make a difference together. We offer a portfolio of products within sample management, liquid handling, and multiplexed detection that minimize assay volumes, reduce material handling costs and put the discovery tools back in the hands of the scientist.
At the Heart of What We Do
Many of our innovations have been born out of the desire to create solutions to existing customer problems; and it’s this ethos that drives SPT Labtech’s R&D efforts. Our strengths come from the trust our customers have with us to develop truly unique, automated technologies to meet their needs. We combine cutting edge science with first-rate engineering to put customers at the heart of everything we do.
A Problem-Solving State of Mind
The substantial breadth of expertise within our company enables us to be involved in the full life cycle of our products from the initial design concept, mechanical and software engineering and prototyping, to final manufacture and sale. These qualities allow us to offer the best possible technical and mechanical support to all the equipment that we supply, hence maintaining excellent client relationships.
Sponsored Content Policy: AZoLifeScience.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.