3D cell cultures and organoids are promising for research and development and applications in personalized medicine. However, more automated and high throughput techniques are required to achieve their full potential.
Historically, achieving automation for handling organoids has been difficult for multiple reasons, such as a limited supply of cells to support these models, prohibitive liquid handling dead volumes, difficulties manipulating viscous basement membrane extracts, and setting up highly reproducible 3D cell cultures.
Corning collaborated with SPT Labtech to establish and moderate a focus group to assess the main barriers, pain points, and opportunities in working with 3D cell cultures.
The session aimed to discover the participants’ experiences in this area to better understand and advise future developments. There was also a desire to provide a platform for delegates to exchange knowledge and assist with optimizing their 3D cell culture methods.
Case Study
A short presentation was provided by Hilary Sherman, Senior Application Scientist at Corning Life Sciences, to start the focus group.
Hilary described how the dragonfly® discovery from SPT Labtech had been utilized for the accurate and even dispensing of small (3 µL) droplets of human intestinal organoids mixed with Corning® Matrigel® matrix for organoid culture.
These half-dome cultures were assayed using high-content imaging for physiological alterations after forskolin-induced swelling (FIS), an in vitro assay for examining the response to drugs in cystic fibrosis patients.
After this presentation, participants were encouraged to share their insights on three main areas: the pain points of using 3D cells, the participant’s needs regarding cell culture optimization, and trade-offs when automating parts of the process.
Current Pain Points Regarding Setting up 3D Cultures
1. Organoid Disaggregation and Scaling Throughput
The disaggregation and re-seeding into 3D cultures is a recognized challenge when employing organoids, and several focus group participants shared this.
It appears to be likely that the fragility of the organoids does not work well with automated cell plating.
As verified by survey responses, there is a strong interest in boosting the throughput of 3D cell culture applications. A straightforward solution to attaining this higher throughput is using assay miniaturization.
The speed at which it is possible to fill a 1536-well plate was discussed and it was noted that a non-contact dispenser, such as the dragonfly discovery, delivers a more rapid rate than contact pipetting.
Holly Hung, Field Application Scientist at SPT Labtech, explained how a single syringe dispense can fill a 1536-well plate in approximately eight minutes while reducing dead volumes to only 30 uL per channel when employing the dragonfly discovery dispenser.
Although even quicker dispense times may be attained with multiple channels, the extra dead volume required may only be reasonable in the highest throughput circumstances.
2. Maintaining Cool Temperatures
Keeping materials cool when working with Matrigel-embedded 3D cell cultures was mentioned as a concern. The positive displacement technology provided by SPT Labtech facilitates simpler dispensing of viscous solutions, but maintaining strict temperature control is still vital.
As mentioned in the case study example, Corning and SPT Labtech addressed this concern by pre-chilling dispense syringes and utilizing passive chill blocks to keep reagents cold on the reservoir tray.
Anne Hammerstein, Product Manager at SPT Labtech, described a prototype in development with SPT Labtech that may improve the chilling functionality.
This involves using a ‘wine cooler’-style design that surrounds the syringe body and helps maintain a cool reagent temperature between reagent aspiration and dispensing steps.
A cool temperature is more difficult when multiple plates must be processed. Although utilizing a cold room environment may be feasible when imagining a very high-throughput setup, this does propose challenges.
Such conditions are uncomfortable for the operator and are usually avoided. Taking instruments in and out of cold room conditions is also not advised as it risks damage to parts via condensation.
A combination of positive displacement technology, rapid dispense times, and passive cooling elements provides the best chance of reproducible and accurate dispensing of temperature-sensitive materials, such as Matrigel.
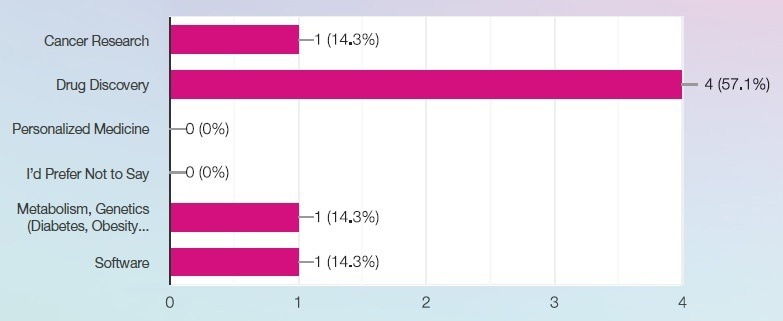
What best describes your primary area of research or development? Image Credit: SPT Labtech
Throughput for 3D Assays
Participants were asked:
- What kind of throughput would be desirable for your 3D assays?
- Do you have to compromise on model strength for throughput/cost considerations?
These questions stimulated a discussion about how the acceptable compromises and trade-offs largely depended on the application.
When utilizing organoids for drug screening and imaging purposes, any deviation in size and the number of organoids per droplet is likely not a vital issue since the high-content analysis can normalize assay readouts to the organoid number.
For this application, the consistency and location of the droplets are crucial to ensure precise and rapid imaging. However, working with an ATP-luminescence readout, organoids' number, consistency, and relative size are critical, and automation to provide standardization is necessary.
A certain extent of variability is expected when setting up 3D cultures, partly because of the biological sample’s heterogeneity. However, while acknowledging this, it is vital not to add more technical variance as it may disguise real differences between, for example, control and treatment wells.
Utilization of positive displacement dispensing facilitates robust control over the volumes, reducing technical variance. As noted in the discussion concerning maintaining a cool temperature, the case study demonstrated that plate variability (measured by %CV) usually increases after dispensing two plates.
The new syringe chiller should alleviate this impact by supplying passive cooling of the Matrigel reagent for more prolonged periods.
The best practice for automating the dispensing of several cell lines was enquired about, regarding testing against a panel of drugs. Here, two approaches may be used, dependent on the number of drugs and cell lines involved in the screen.
This may be achieved for small quantities of drugs and cells by laying out different cell lines in different plate sections and providing drug doses to each cell line according to a custom layout.
However, for greater quantities of cell lines, it is better to assign a plate to each cell line and to apply a standardized drug/dose layout (either using acoustic dispensing or stamping from diluted assay-ready plates).
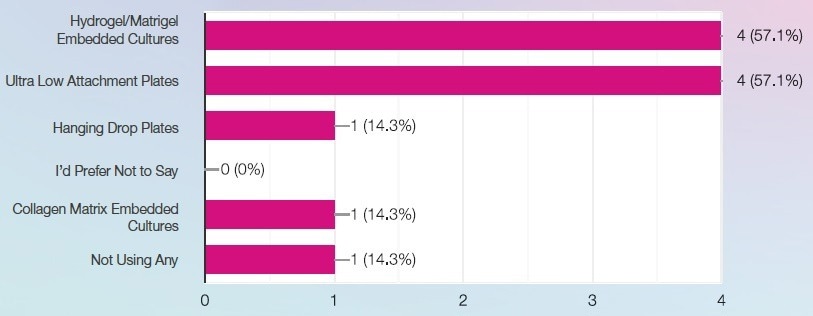
What 3D cell formats does your laboratory currently utilize? Image Credit: SPT Labtech
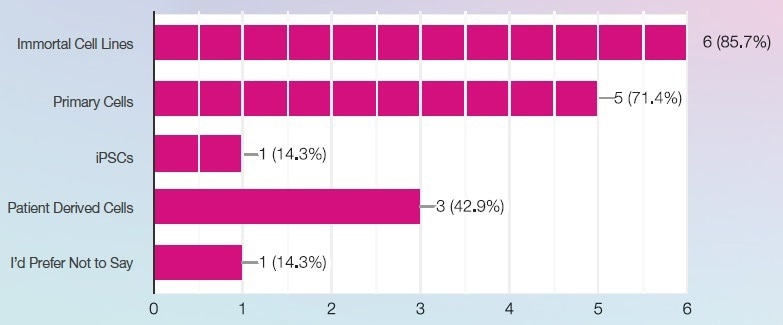
What source of cells do you work with? Image Credit: SPT Labtech
Other Liquid Handling Pain Points in Laboratories
Advances in liquid handling technology would be advantageous for exchanging the cell culture media in screening wells. Working with slow-metabolizing compounds in toxicology applications may require several days to observe an impact on the organoid.
During this time, the media in the wells requires refreshment to expand the screening window and provide time to measure the effect of compounds.
It is highly technically challenging to exchange the media on the plates without compromising the cell culture, meaning automation to facilitate refreshing the media and extending the screening assay would be appreciated.
Participants mentioned some technologies designed to overcome this challenge, such as Biotek’s plate washer that aspirates and adds fresh media.
Organoids are usually predictable in their location in the well, so setting automation to aspirate at a certain speed and height can effectively remove the cell culture media without disturbing the spheroid.
The addition of fresh media momentarily disturbs the 3D object, but gravity eventually repairs its position.
A NanoShuttle employs a magnetizing technology that makes the addition and removal of the solution more straightforward by holding the 3D cells in a motionless position during aspiration.
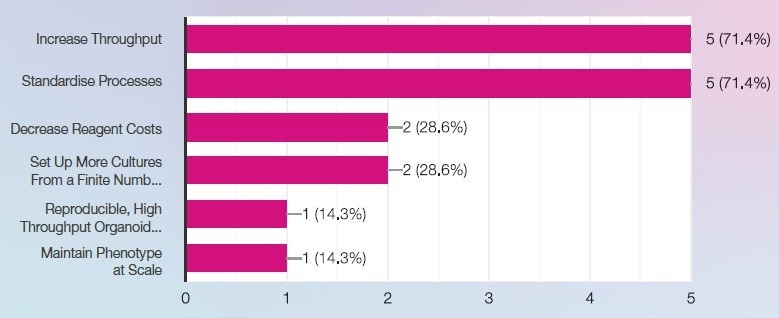
What best describes your laboratory’s 3D cell culture goals? Image Credit: SPT Labtech
Optimization of 3D Cell Culture Conditions
Participants were asked if they performed any optimizations of their 3D cell culture conditions. The conversation then involved how Design of Experiments (DOE) could assist with optimizing cell culture conditions. One variable that demands optimization for any 3D cell culture is how much the media requires aspiration and at what point.
Other variables which may need optimization are the number and ratio of cell types and the concentration of Matrigel. Using scarce patient-derived cells may not allow all variables to be systematically tested one factor at a time, so in these cases, a statistical method can help to narrow down the number of options.
How many plates (96/384) of 3D cell cultures does your laboratory currently process per week? Image Credit: SPT Labtech
Summary
The discussion indicated that application is fundamental when contemplating automation and optimization for 3D cell cultures. Typical solutions are likely to include semi-automation that demands some level of user intervention compared to full end-to-end automation.
However, there are opportunities to realize process efficiencies through the use of assay miniaturization as well as streamlining 3D cell culture workflows via reliable and rapid dispensing of reagents.
Acknowledgments
Produced from materials originally authored by SPT Labtech. The original authors wish to thank the participants for providing their time and insights during this focus group.
The leaders of the focus group were: Anne Hammerstein (AFH) Product Manager at SPT Labtech who acted as Moderator, Hilary Sherman (HS) Senior Application Scientist at Corning who acted as Presenter, and Holly Hung (HH) Field Application Scientist at SPT Labtech who acted as Co-Presenter.
About SPT Labtech
We Design and Manufacture Robust, Reliable and Easy-to-Use Solutions for Life Science
We enable life scientists through collaboration, deep application knowledge, and leading engineering to accelerate research and make a difference together. We offer a portfolio of products within sample management, liquid handling, and multiplexed detection that minimize assay volumes, reduce material handling costs and put the discovery tools back in the hands of the scientist.
At the Heart of What We Do
Many of our innovations have been born out of the desire to create solutions to existing customer problems; and it’s this ethos that drives SPT Labtech’s R&D efforts. Our strengths come from the trust our customers have with us to develop truly unique, automated technologies to meet their needs. We combine cutting edge science with first-rate engineering to put customers at the heart of everything we do.
A Problem-Solving State of Mind
The substantial breadth of expertise within our company enables us to be involved in the full life cycle of our products from the initial design concept, mechanical and software engineering and prototyping, to final manufacture and sale. These qualities allow us to offer the best possible technical and mechanical support to all the equipment that we supply, hence maintaining excellent client relationships.
Sponsored Content Policy: AZoLifeScience.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.