Laboratory Developed Tests (LDTs) are specialized diagnostic tests designed, manufactured, and employed within a single laboratory. These tests serve a vital role in healthcare, allowing rapid and specialized diagnostics to be conducted for a wide range of targets such as proteins, RNA, and DNA.
LDTs are necessary when commercial tests are impractical or unavailable, such as for the diagnosis of rare diseases and for facilitating personalized medicine.
LDTs must be subjected to rigorous validation to guarantee accuracy because they are crucial in guiding treatment decisions. This accuracy makes LDTs indispensable in modern healthcare.
The Role of LDTs in Personalized Cancer Treatment
LDTs that utilize Next-Generation Sequencing (NGS) have gained great significance as companion diagnostics for personalized cancer care. These NGS-driven LDTs identify specific genetic markers in patients, allowing healthcare providers to tailor treatments more effectively.
NGS technology facilitates the assessment of multiple genes in a single assay, eliminating the need for multiple tests and delivering a faster, cheaper diagnostic solution while extending the utility of valuable clinical samples.
Automated Liquid Handling Boosts the Precision and Efficiency of LDTs
Automated liquid handling solutions are revolutionizing how labs conduct NGS-driven LDTs by significantly improving efficiency and accuracy. These automated systems can help labs to meet ambitious Key Performance Indicators (KPIs) cost-effectively.
Automated liquid handling also minimizes human error by automating laborious and repetitive pipetting tasks. This frees up skilled personnel to carry out other crucial tasks, facilitating more efficient and streamlined workflows and minimizing turnaround times, getting results to patients faster.
Automation also enables labs to easily scale their operations to satisfy future testing demands. This allows the labs to adapt to the ever-changing fields of personalized medicine and cancer care.
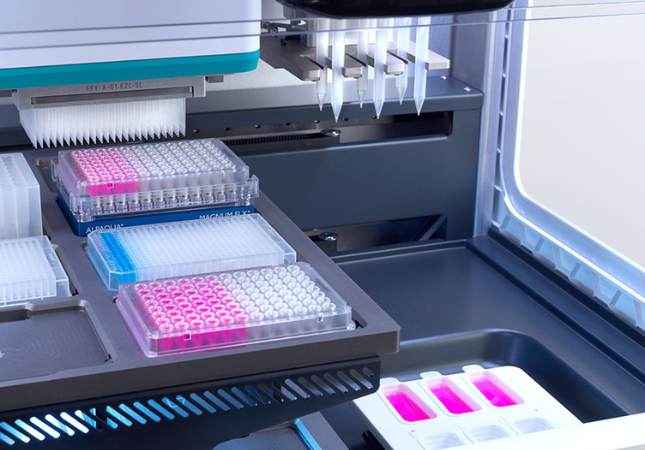
Image Credit: SPT Labtech
Important Considerations when Choosing an Automated Liquid Handling Solution for LDTs
Labs operating at high throughput face pressure to maintain quality while meeting regulatory requirements. Selecting an automated liquid handling solution for LDTs demands thoughtful consideration.
These considerations include the following:
- Access to service and support: The level of technical support and maintenance services the vendor offers should be considered. Access to sufficient support is vital to minimize downtime and guarantee the longevity and reliability of the system.
- Ease of use: An intricate and difficult-to-use system can slow operations, increasing the likelihood of errors. It is crucial to have a user-friendly interface and ease of operation for efficiency.
- Regulatory compliance: Any new system must satisfy all LDT regulatory requirements. These may include specific data reporting and quality control features.
- Versatility: As LDTs continue to develop, a flexible system that can adapt to new techniques and assays is highly advantageous. This is especially important for labs that plan to expand into new areas of testing.
Conclusion
In Laboratory Developed Tests (LDTs), automated liquid handling systems are proving to be a transformative technology, especially for LDTs that use Next-Generation Sequencing (NGS) for personalized cancer care.
These systems can enhance accuracy, boost efficiency, and deliver scalable solutions, allowing labs to meet current and future challenges more effectively.
However, due to the high stakes and regulatory rigor associated with LDTs, labs must make well-informed decisions when selecting and implementing automated solutions.
The right system can improve the quality of diagnostics and enable adaptability in a rapidly advancing healthcare environment.
References and Further Reading
- FDA 2023, Laboratory Developed Tests, FDA https://www.fda.gov/medical-devices/in-vitro-diagnostics/laboratory-developed-tests
- Pallavi Upadhyay PhD 2022, Current Challenges Facing Lab Developed Tests, Today’s Clinical Lab https://www.clinicallab.com/trends/clinical-lab-business-management/current-challenges-facing-lab-developed-tests-26502
About SPT Labtech
SPT Labtech designs and manufactures robust, reliable and easy-to-use solutions for life science. We enable life scientists through collaboration, deep application knowledge, and leading engineering to accelerate research and make a difference together. We offer a portfolio of products within sample management, liquid handling, and multiplexed detection that minimize assay volumes, reduce material handling costs and put the discovery tools back in the hands of the scientist.
At the Heart of What We Do
Many of our innovations have been born out of the desire to create solutions to existing customer problems; and it’s this ethos that drives SPT Labtech’s R&D efforts. Our strengths come from the trust our customers have with us to develop truly unique, automated technologies to meet their needs. We combine cutting edge science with first-rate engineering to put customers at the heart of everything we do.
A Problem-Solving State of Mind
The substantial breadth of expertise within our company enables us to be involved in the full life cycle of our products from the initial design concept, mechanical and software engineering and prototyping, to final manufacture and sale. These qualities allow us to offer the best possible technical and mechanical support to all the equipment that we supply, hence maintaining excellent client relationships.
Sponsored Content Policy: AZO Life Sciences publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of AZO Life Sciences which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.