By Chi Chi ChengReviewed by Lauren Hardaker
Uncover how cutting-edge microbiome diagnostics and AI technologies are redefining early disease detection, revealing how our gut microbes, as well as those on our tongues, hold the key to predicting and preventing illness.
 Image credit: New Africa/Shutterstock.com
Image credit: New Africa/Shutterstock.com
Introduction
The human microbiome, including that of the skin, gut, and oral cavity, serves as a multi-faceted indicator of physiological and mental health. With the rising prevalence of chronic illnesses and the limitations of conventional diagnostics, exploring the microbiome offers new opportunities for earlier, less invasive, and more precise health monitoring. Advances in metagenomic sequencing have been central to this development, enabling comprehensive profiling of microbial communities beyond the limits of culture-based methods.1,2
Recent high-resolution metagenomic analyses now permit the detection of both bacterial and viral genome signatures (metagenome-assembled genomes, MAGs), providing multi-kingdom insights into disease-related microbial dynamics. By capturing taxonomic diversity, functional genes, spatial organization, and microbial interactions, metagenomics offers a powerful framework for explaining the relationships between microbiome alterations and disease development, particularly in chronic and neurodegenerative conditions.1
Such integrative microbial data, when combined with AI-driven analysis, are increasingly applied in diagnostic modeling to distinguish health states in disorders like autism spectrum disorder and Parkinson’s disease. These approaches are reshaping the diagnostic landscape and offer opportunities for precision medicine applications across diverse medical and research settings. This article highlights the emerging potential of microbiome diagnostics, focusing on the gut-brain axis and innovations leveraging artificial intelligence and machine learning.1,2,4,9
DISCOVER HOW THE MICROBIOME CAN IMPACT DISEASE DIAGNOSTICS. DOWNLOAD YOUR PDF COPY NOW!
Current Limitations of Conventional Diagnostics
Conventional diagnostics often fall short in the timely detection of chronic diseases that may evolve into complex conditions. For instance, pancreatic cancer, among the most aggressive malignancies, is increasingly affecting younger populations and remains difficult to detect early, as symptoms are often nonspecific and biomarkers are unreliable.1 Recent reviews emphasize that the tumor microenvironment in pancreatic cancer is profoundly shaped by the microbiome, where microbial inflammation promotes oncogenic signaling and suppresses antitumor immunity, indicating potential for microbiome-guided screening and immunotherapy design.10
For neurodegenerative and psychiatric disorders, such as Parkinson’s disease, schizophrenia, and autism spectrum disorder, diagnostics rely largely on symptom-based approaches that overlook biological underpinnings, which would enable earlier intervention. Emerging microbiome data show that specific microbial signatures in fecal or oral samples correlate with symptom severity and neuroinflammatory markers, supporting their value as adjunctive diagnostic tools. Thus, conventional reactive diagnostics overlook growing evidence that lifestyle, diet, and the gut microbiome significantly influence disease risk and progression.1,2,3,9
Gut-Brain Axis and the Role of the Microbiome
A major area of current research involves understanding how the gut microbiota impacts brain function. The gut-brain axis is a bidirectional communication network that integrates neural pathways, including the enteric nervous system, the vagus nerve, sympathetic and spinal nerves, and humoral pathways mediated by cytokines, hormones, and neuropeptides.2,5 Within this system, microbes act as key modulators by influencing bottom-up signaling through metabolites, neurotransmitters, and immune interactions. Microbial metabolites, such as short-chain fatty acids (SCFAs) and tryptophan derivatives, regulate neurotransmitter synthesis and can alter serotonergic and dopaminergic signaling, which are critical for cognition and emotional balance.2,5,8
Notably, approximately 90 % of serotonin is produced in the gut, rather than in the brain. The microbiome regulates serotonin by synthesizing it directly, producing SCFAs that stimulate its production, and altering tryptophan availability, the precursor of serotonin. A diverse microbiome supports balanced serotonin levels, influencing digestion, immunity, mood, and cognition.3,8
The vagus nerve, the central “superhighway” of the gut-brain axis, plays a pivotal role in transmitting microbial signals to the brain and regulating parasympathetic responses. Dysregulation in this system, including stress-induced cortisol release, can impair neurogenesis, which is shown to be microbiome-dependent. Experimental studies in germ-free mice confirm that the absence of gut microbes reduces hippocampal neurogenesis, linking microbial composition to learning and stress resilience.2,4
These findings highlight the microbiome’s influence on stress regulation, cognition, and overall brain health, positioning it as a promising target for both diagnostic and therapeutic purposes.
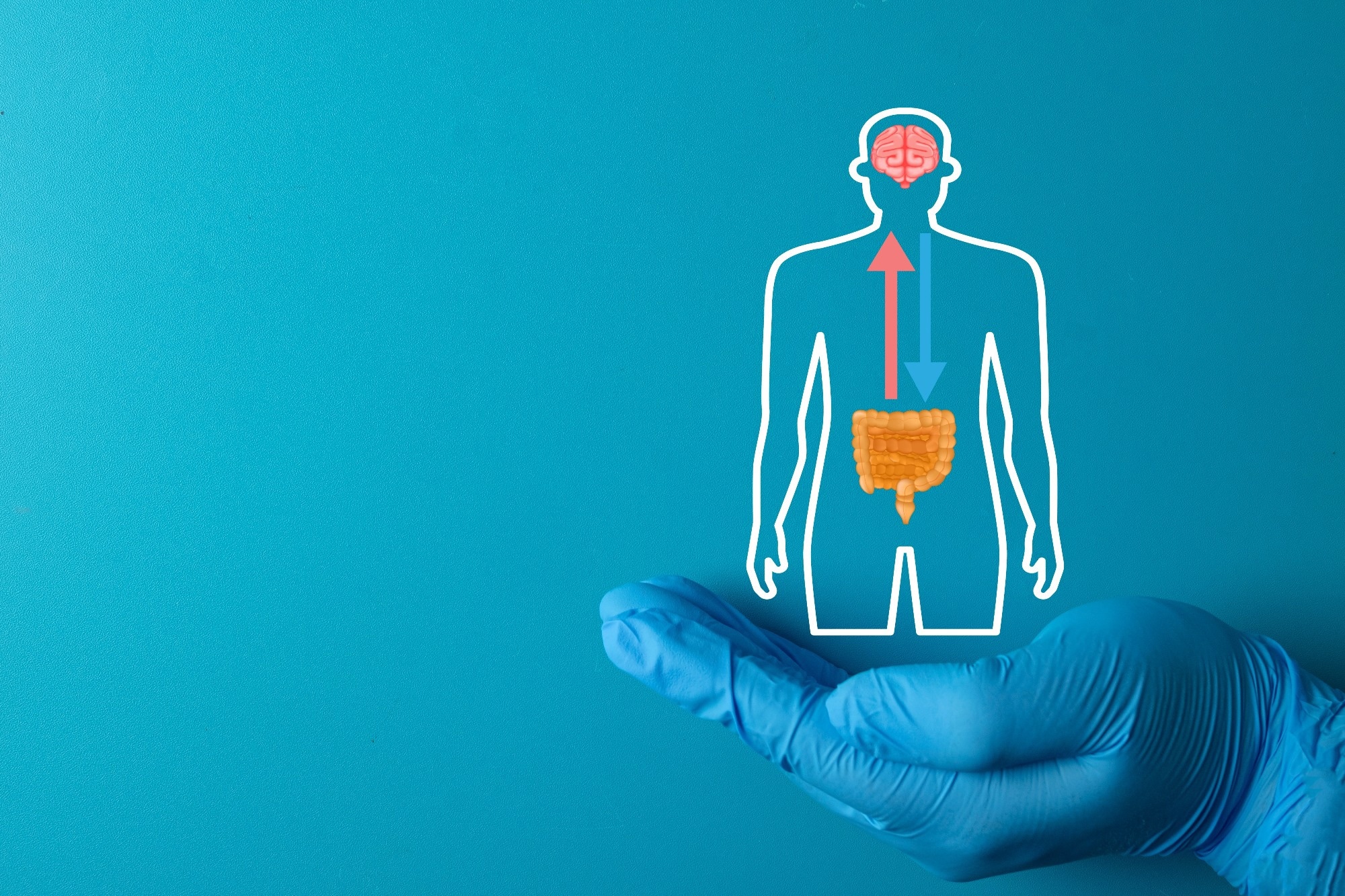 Image credit: Toey Andante/Shutterstock.com
Image credit: Toey Andante/Shutterstock.com
Gut Microbiome Diagnostics for Early Disease Detection
Gut-related Diagnostics
Gut-related conditions, including pancreatic cancer, metabolic disorders, and liver diseases, are closely tied to microbiome composition and activity. In pancreatic cancer, the tumor microenvironment is shaped by microbe-induced inflammation, which promotes oncogenic signaling and suppresses immune responses. Due to symptoms often being nonspecific and screening tools being inadequate, microbiome profiling offers a promising early detection strategy. Recent reviews have highlighted that microbiome-based biomarkers, when combined with liquid biopsies, could enhance risk stratification and treatment outcomes in pancreatic cancer immunotherapy.1,10
Brain-related Diagnostics
The gut microbiome is increasingly recognized as a modifier of neurodegenerative and psychiatric disorders. In Parkinson’s disease, gastrointestinal symptoms often precede motor dysfunction, suggesting early microbiome involvement.
A large-scale meta-analysis revealed consistent microbial alterations in patients with Parkinson’s disease, including enrichment of Lactobacillus, Akkermansia, and Bifidobacterium, and depletion of SCFA-producing taxa such as Faecalibacterium and Lachnospiraceae, which are linked to intestinal inflammation and α-synuclein aggregation.9
In autism spectrum disorder, panels of bacterial taxa and microbial genes have shown diagnostic promise in machine-learning models. A recent fecal microbiome study involving 598 children identified combined bacterial and viral genome markers capable of distinguishing ASD from controls with AUROC scores up to 0.886, demonstrating potential for noninvasive diagnostic screening. Schizophrenia research also reveals gut microbiome alterations tied to immune dysregulation, though further validation is needed.1,3
Overall, these findings suggest that microbiome profiling could complement conventional diagnostics, offering earlier and more precise insights into brain-related disorders and their underlying biological mechanisms.
Potential Role of the Oral Microbiome for Early Disease Detection
The oral microbiome, particularly that of the tongue, is emerging as a valuable diagnostic window into systemic health. Evidence from DNA sequencing indicates that most oral microbes are site specialists, adapted to specific niches within the mouth. High-resolution imaging studies of the tongue dorsum biofilm have revealed a striking spatial organization, e.g., Streptococcus species clustering at the periphery and Actinomyces near the epithelial core, suggesting structured ecological interactions. This spatial ecology underpins disease-associated shifts in microbial communities and supports the use of spatially resolved oral diagnostics.5
Recent advances in AI-powered tongue scanning enable noninvasive diagnostics and monitoring. For example, tongue color imaging has been linked to stress levels, while distinct microbiome profiles of the tongue coating are associated with type 2 diabetes. Studies show that yellow tongue coating in T2D correlates with elevated Lactobacillus abundance and altered glucose metabolism, suggesting that tongue microbiota reflect systemic metabolic states.6,7
Intelligent image analysis combined with microbiome profiling has shown success in diagnosing metabolic-associated fatty liver disease (MAFLD). Integrative AI-microbiome models combining tongue image data, clinical metrics, and bacterial markers, such as Streptococcus and Rothia, achieved diagnostic accuracies above 96 %, underscoring their clinical potential. Together, these findings suggest that the tongue may soon serve as a rapid, accessible platform for continuous health monitoring.8
Download your PDF copy now!
Future Perspectives
Although still in early development, microbiome diagnostics hold significant promise for transforming the healthcare industry. As AI and machine learning continue to advance rapidly, integrating microbiome data could enhance predictive modeling for chronic diseases, improve personalized preventive strategies, and facilitate continuous self-monitoring. In particular, AI-enhanced tongue and gut microbiome diagnostics represent accessible, noninvasive, and data-rich platforms for real-time health assessment.6,7,8
Moving forward, refining our understanding of spatial microbiome structures and the ecological connections between the gut and brain will be key for validating biomarkers across populations and conditions. As Voltaire once wrote, “we must cultivate our garden”, a reminder that understanding and tending our microbial ecosystems may indeed be central to sustaining human health.
References
- Vitiello GA, Cohen DJ, Miller G. Harnessing the Microbiome for Pancreatic Cancer Immunotherapy. Trends in Cancer. 2019;5(11):670-676. DOI:10.1016/j.trecan.2019.10.005, https://www.cell.com/trends/cancer/abstract/S2405-8033(19)30198-0
- Bercik P, Collins SM, Verdu EF. Microbes and the gut-brain axis. Neurogastroenterology & Motility. 2012;24(5):405-413. DOI:10.1111/j.1365-2982.2012.01906.x, https://onlinelibrary.wiley.com/doi/10.1111/j.1365-2982.2012.01906.x
- Chen Y, Xu J, Chen Y. Regulation of Neurotransmitters by the Gut Microbiota and Effects on Cognition in Neurological Disorders. Nutrients. 2021;13(6):2099. DOI:10.3390/nu13062099, https://www.mdpi.com/2072-6643/13/6/2099
- Ogbonnaya ES, Clarke G, Shanahan F, Dinan TG, Cryan JF, O’Leary OF. Adult Hippocampal Neurogenesis Is Regulated by the Microbiome. Biological Psychiatry. 2015;78(4):e7-e9. DOI:10.1016/j.biopsych.2014.12.023, https://www.biologicalpsychiatryjournal.com/article/S0006-3223(15)00007-4/abstract
- Wilbert SA, Welch JLM, Borisy GG. Spatial Ecology of the Human Tongue Dorsum Microbiome.Cell Reports. 2020;30(12):4003-4015.e3. DOI:10.1016/j.celrep.2020.02.097, https://www.cell.com/cell-reports/fulltext/S2211-1247(20)30271-0
- Hernandez J, Ferguson C, Sano A, et al. Stress measurement from tongue color imaging. In: 2017 Seventh International Conference on Affective Computing and Intelligent Interaction (ACII). 2017:152-157. DOI:10.1109/ACII.2017.8273593, https://ieeexplore.ieee.org/document/8273593
- Wang Y, Li J, Hu H, et al. Distinct microbiome of tongue coating and gut in type 2 diabetes with yellow tongue coating. Heliyon. 2024;10(1):e22615. DOI:10.1016/j.heliyon.2023.e22615, https://www.sciencedirect.com/science/article/pii/S2405844023098237
- Dai S, Guo X, Liu S, et al. Application of intelligent tongue image analysis in Conjunction with microbiomes in the diagnosis of MAFLD. Heliyon. 2024;10(7):e29269. DOI:10.1016/j.heliyon.2024.e29269, https://www.sciencedirect.com/science/article/pii/S2405844024007696
- Romano S, Savva GM, Bedarf JR, Charles IG, Hildebrand F, Narbad A. Meta-analysis of the Parkinson’s disease gut microbiome suggests alterations linked to intestinal inflammation. npj Parkinsons Dis. 2021;7(1):27. DOI:10.1038/s41531-021-00156-z, https://www.nature.com/articles/s41531-021-00156-z
- Wan Y, Wong OWH, Tun HM, et al. Fecal microbial marker panel for aiding diagnosis of autism spectrum disorders. Gut Microbes. 2024;16(1):2418984. DOI:10.1080/19490976.2024.2418984, https://www.tandfonline.com/doi/full/10.1080/19490976.2024.2418984
Last Updated: Jan 8, 2026