By Dr. Said QabbaahReviewed by Lauren Hardaker
This article examines how advances in AI, multi-omics integration, genome editing, and digital infrastructure are reshaping life sciences and biotechnology as they move into 2026. It highlights the scientific, clinical, and operational trends that are accelerating translation while introducing new regulatory and data challenges.
 Image credit: IM Imagery/Shutterstock.com
Image credit: IM Imagery/Shutterstock.com
As life sciences and biotechnology enter 2026, the field continues to evolve through rapid technological and scientific advances. Innovations that were once experimental are now embedded across research, development, and commercialization, driving real-world impact and shaping the industry in the year ahead.
Where Biology Meets Technology
A defining feature of contemporary life sciences is the growing convergence of biological data and computational methods. High-throughput biology increasingly intersects with machine learning and multimodal integration approaches, deepening biological insight and supporting scientific discovery.1
Academic–industry partnerships, shared research infrastructure, and open-data initiatives are accelerating this integration by improving access to high-quality data and enabling larger-scale inquiry. For example, the Netherlands X-omics Findable, Accessible, Interoperable, and Reusable (FAIR) Data Cube provides a federated platform for omics datasets, facilitating cross-functional collaboration.2
Recent evaluations emphasize that federated FAIR infrastructures not only improve data reuse but also address privacy, data sovereignty, and regulatory constraints by enabling computation to be brought to the data rather than centralized data pooling.2
Organizations that incorporate such strategies within their operating models are better positioned to translate complex scientific insights into practical applications by leveraging shared resources and interdisciplinary expertise.1,2
To explore the biotech trends shaping 2026 download your free PDF copy here
AI-Driven Drug Discovery
Artificial intelligence (AI) is now an established component of modern drug discovery pipelines. Its role is expected to expand beyond target identification to support integrated decision-making across the entire drug development lifecycle, including lead optimization, biomarker discovery, and patient stratification.
Foundation models trained on extensive biological and clinical datasets are expediting predictions of protein structures, ligand binding, and drug-like properties. These computational tools are becoming routinely integrated with experimental platforms, such as functional genomics, to accelerate hypothesis testing and early-stage drug development.1,3
Systematic analyses indicate that AI adoption has its greatest measurable impact in preclinical and IND-enabling stages, where it compresses discovery timelines and improves hit-to-lead efficiency. At the same time, later-stage clinical applications remain constrained by validation, explainability, and regulatory expectations.
By leveraging omics data, real-world evidence, and electronic health records, AI is enhancing translational applications by stratifying patient populations with higher predicted responsiveness to targeted therapies. However, model performance remains tightly coupled to data quality, representativeness, and integration strategies rather than algorithmic sophistication alone.3
Advancing Gene Editing and Cell Therapies
Gene editing technologies are undergoing constant evolution, with refinement of CRISPR-based systems and the emergence of base and prime editors. Research increasingly focuses on enhancing specificity and delivery efficiency in vivo using engineered vectors and optimized nuclease platforms, as well as improving the stability of genome edits.4
Therapeutic genome editing modalities, such as recombinant adeno-associated virus (rAAV)-mediated delivery and lipid nanoparticles (LNPs), are expanding the clinical applicability of CRISPR-based therapies by addressing key aspects of therapeutic efficacy and safety.4,5
Recent reviews highlight next-generation biological delivery platforms, including engineered viral vectors, virus-like particles, and mammalian-derived nanoparticles, as critical to enabling efficient in vivo genome editing while reducing immunogenicity and off-target risk.5 Within rAAV-based CRISPR delivery, innovations such as compact Cas orthologs, dual-vector strategies, and trans-splicing approaches are mitigating payload size limitations. However, durability of expression, immune responses, and re-dosing constraints remain key translational challenges.4
Advances in vector engineering, including refined viral vectors and emerging non-viral platforms, are helping overcome delivery challenges and enhancing the clinical translation of gene-based therapies. Genome-edited allogeneic CAR-T cells exemplify this progress, offering scalable “off-the-shelf” immunotherapies while introducing new safety, immunogenicity, and manufacturing considerations that remain active areas of clinical investigation.5,6
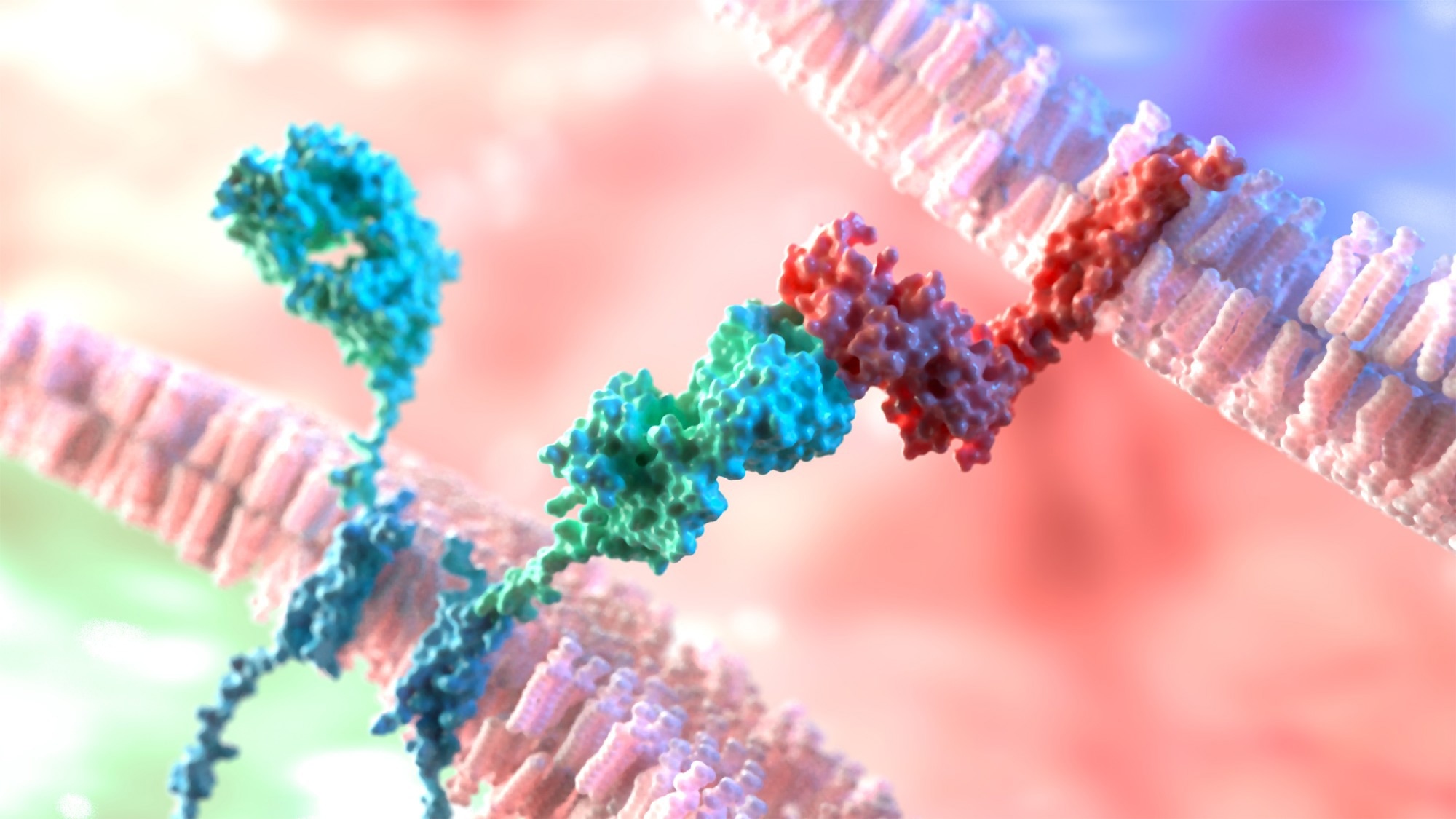 Image credit: Alpha Tauri 3D Graphics/Shutterstock.com
Image credit: Alpha Tauri 3D Graphics/Shutterstock.com
Evolving Spatial and Single-Cell Biology
The focus of spatial transcriptomics and single-cell analysis is shifting from purely technology development toward standardization, multimodal integration, and computational frameworks that enhance reproducibility and cross-platform interpretability.
Single-cell multi-omics has evolved to the point where integrating transcriptomic, epigenomic, proteomic, and spatial imaging data enables comprehensive exploration of cellular heterogeneity and mechanistic pathways at high resolution. Methodological advances increasingly rely on deep generative and foundation models to address batch effects, missing modalities, and cross-platform heterogeneity, enabling scalable joint embeddings across diverse single-cell and spatial datasets.1,7
These advanced technological capabilities now allow simultaneous measurement of gene expression, protein abundance, and cellular context, producing high-dimensional datasets that guide target validation, uncover biomarkers, and investigate mechanisms previously inaccessible with single-modality approaches.7
As spatial and single-cell transcriptomics datasets increase in scale and complexity, automated methods for aligning and integrating data across tissue slices will be essential for accurate downstream analysis, with their adoption reliant on standardized computational tools, reproducibility, and efficient analytical frameworks.8
Next-Generation Diagnostics and Precision Medicine
Emerging diagnostics, including molecular assays, liquid biopsy, and multi-analyte platforms, are developing rapidly, enabling earlier disease detection, more precise risk stratification, and better-informed therapeutic decisions, while also supporting ongoing treatment monitoring.9,10
In oncology, cell-free DNA (cfDNA)-based liquid biopsy approaches are proving particularly valuable for sensitive diagnosis and characterization, with growing evidence supporting their use across cancer care. These approaches are also gaining traction across additional clinical areas, including transplant surveillance, infectious disease detection, and chronic disease management, where repeated tissue sampling is impractical.9,10
By integrating multi-layer molecular profiling, advanced diagnostics generate rich datasets that capture disease trajectories and inform precision medicine. Regulatory guidance increasingly emphasizes the concurrent development of targeted therapies and companion diagnostics, while also acknowledging the need for validation flexibility when clinical samples are limited, particularly for rare biomarkers.1,11
Decentralized and Digitally Enabled Clinical Trials
Decentralized clinical trials are evolving beyond pilot implementations toward broader hybrid models that combine on-site assessments with remote monitoring, digital endpoints, and home-based data collection. This shift is driven by the growing adoption of wearable technologies and digital health platforms, which enable continuous, patient-focused data capture and extend observation into the community. This improves trial participation, adherence, and retention compared with traditional designs.12
Recent qualitative studies indicate that while decentralized models improve accessibility, they may also introduce new burdens for clinical research staff and risk weakening patient–provider relationships if not carefully designed.12
Decentralized Clinical Trials and Patient Needs
Video credit: parexel/Youtube.com
Biomanufacturing and Automation
Biomanufacturing, a critical bottleneck for many advanced therapies, is advancing through increased automation, modular production models, and digitally enabled process control, supporting greater efficiency, scalability, and operational consistency. Progress is reinforced through the deployment of continuous manufacturing, single-use technologies, and automated workflows, thereby reducing contamination risk and enhancing process quality. Digital twins are increasingly applied to enable real-time monitoring, predictive maintenance, and data-driven optimization across development and manufacturing workflows.13
These innovations collectively strengthen manufacturing robustness and capacity, positioning organizations to support late-stage development and the transition toward commercialization while ensuring reliable product performance and regulatory compliance.13
Direct-to-Consumer and Commercial Strategies
Direct-to-consumer (DTC) digital health models are expanding rapidly, offering home-based testing, telemedicine, mobile health tools, and other consumer-facing services that engage individuals beyond traditional healthcare settings.14
With the availability of large volumes of patient-generated data and improvements in access and convenience in preventive care, DTC platforms support the generation of real-world evidence to inform product development, patient support strategies, and outcomes research. However, recent analyses highlight uneven population coverage and persistent equity gaps, underscoring the need for systematic monitoring of access and impact.14,15
Looking Ahead to 2026 and Beyond
The life science and biotechnology landscape in 2026 presents both unprecedented opportunity and rising complexity. Success will demand more than technical expertise; researchers and industry professionals must combine data literacy, operational rigor, and a nuanced understanding of regulatory and market dynamics. As these trends continue to unfold, strategic collaboration and evidence-driven adaptation will be essential to sustaining meaningful innovation.
References
- Baião, A. R., Cai, Z., Poulos, R. C., Robinson, P. J., Reddel, R. R., Zhong, Q., Vinga, S., & Gonçalves, E. (2025). A technical review of multi-omics data integration methods: from classical statistical to deep generative approaches. Briefings in Bioinformatics, 26(4), bbaf355. DOI:10.1093/bib/bbaf355, https://academic.oup.com/bib/article/26/4/bbaf355/8220754
- Liao, X., Ederveen, T. H. A., Niehues, A., de Visser, C., Huang, J., Badmus, F., Doornbos, C., Orlova, Y., Kulkarni, P., van der Velde, K. J., Swertz, M. A., Brandt, M., van Gool, A. J., & ’t Hoen, P. A. C. (2024). FAIR Data Cube, a FAIR data infrastructure for integrated multi-omics data analysis. Journal of Biomedical Semantics, 15, 20. DOI:10.1186/s13326-024-00321-2, https://jbiomedsem.biomedcentral.com/articles/10.1186/s13326-024-00321-2
- Dermawan, D., & Alotaiq, N. (2025). From Lab to Clinic: How Artificial Intelligence (AI) Is Reshaping Drug Discovery Timelines and Industry Outcomes. Pharmaceuticals, 18(7), 981. DOI:10.3390/ph18070981, https://www.mdpi.com/1424-8247/18/7/981
- Gil, J.-S., Lee, S., & Koo, T. (2025). Therapeutic in vivo genome editing: Innovations and challenges in rAAV vector-based CRISPR delivery. Gene Therapy. DOI:10.1038/s41434-025-00573-2, https://www.nature.com/articles/s41434-025-00573-2
- Leclerc, D., Siroky, M. D., & Miller, S. M. (2024). Next-generation biological vector platforms for in vivo delivery of genome editing agents. Current Opinion in Biotechnology, 85, 103040. DOI:10.1016/j.copbio.2023.103040, https://www.sciencedirect.com/science/article/abs/pii/S0958166923001507
- Su, J., Zeng, Y., Song, Z., Liu, Y., Ou, K., Wu, Y., Huang, M., Li, Y., & Tu, S. (2025). Genome-edited allogeneic CAR-T cells: the next generation of cancer immunotherapies. Journal of Hematology & Oncology, 18(1), 90. DOI:10.1186/s13045-025-01745-8, https://link.springer.com/article/10.1186/s13045-025-01745-8
- Yiu, T., Chen, B., Wang, H., Feng, G., Fu, Q., & Hu, H. (2025). Transformative advances in single-cell omics: a comprehensive review of foundation models, multimodal integration and computational ecosystems. Journal of Translational Medicine, 23, 1176. DOI:10.1186/s12967-025-07091-0, https://link.springer.com/article/10.1186/s12967-025-07091-0
- Khan, M., Arslanturk, S., & Draghici, S. (2025). A comprehensive review of spatial transcriptomics data alignment and integration. Nucleic Acids Research, 53(12), gkaf536. DOI:10.1093/nar/gkaf536, https://academic.oup.com/nar/article/53/12/gkaf536/8174767
- Landon, B. V., Annapragada, A. V., Niknafs, N., Velculescu, V. E., & Anagnostou, V. (2025). Liquid biopsies across the cancer care continuum. Nature Medicine, 31, 4006–4021. DOI:10.1038/s41591-025-04093-9, https://www.nature.com/articles/s41591-025-04093-9
- Loy, C., Ahmann, L., De Vlaminck, I., & Gu, W. (2024). Liquid Biopsy Based on Cell-Free DNA and RNA. Annual Review of Biomedical Engineering, 26, 169–195. DOI:10.1146/annurev-bioeng-110222-111259, https://www.annualreviews.org/content/journals/10.1146/annurev-bioeng-110222-111259
- Andrews, H. S., Collins, G., Navarro-Serer, B., Stewart, M. D., & Allen, J. D. (2025). Companion Diagnostic FDA Review Flexibilities: An Assessment of CDx for NSCLC to Support Aligned Approaches for Validation. Therapeutic Innovation & Regulatory Science, 59, 676–679. DOI:10.1007/s43441-025-00799-7, https://link.springer.com/article/10.1007/s43441-025-00799-7
- Gamble, E., Heavin, C., & Linehan, C. (2025). Adaptation of Clinical Research Staff to Decentralized Clinical Trials and Impacts on the Patient-Centered Experience: Qualitative Interview Study. Journal of Medical Internet Research, 27, e62947. DOI:10.2196/62947, https://www.jmir.org/2025/1/e62947
- Maharjan, R., Kim, N. A., Kim, K. H., & Jeong, S. H. (2025). Transformative roles of digital twins from drug discovery to continuous manufacturing: Pharmaceutical and biopharmaceutical perspectives. International Journal of Pharmaceutics: X, 10, 100409. DOI:10.1016/j.ijpx.2025.100409, https://www.sciencedirect.com/science/article/pii/S2590156725000945
- Nagappan, A., Zhu, X., Moucheraud, C., & Jung, O. S. (2025). Populations and Health Domains Served by Direct-to-Consumer Digital Health Companies in the United States, 2011–2023: Cross-Sectional Study. JMIR Formative Research, 9, e78431. DOI:10.2196/78431, https://formative.jmir.org/2025/1/e78431
- Mahadik, S., Sen, P., & Shah, E. J. (2025). Harnessing digital health technologies and real-world evidence to enhance clinical research and patient outcomes. Digital Health, 11, 20552076251362097. DOI:10.1177/20552076251362097, https://journals.sagepub.com/doi/10.1177/20552076251362097
Last Updated: Jan 22, 2026