Revealing the interactions between metal ions and peptides in the body could help develop enhanced treatments for Alzheimer’s diabetes, and other diseases.
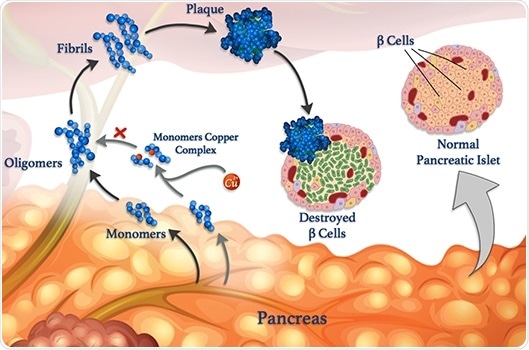
Copper ions (red spheres) can attach to peptide monomers (blue) and prevent them from clumping together to form oligomers, fibrils, and damaging plaques. Image Credit: © 2020 Mawadda Alghrably.
Gaining insights into these interactions is the main aim of a study, co-led by KAUST, that unravels how metals, like copper, can influence the formation of harmful clumps of misfolded peptide clusters named fibrils, which underlie various diseases.
Errant peptides are associated with neurological conditions like Alzheimer’s, and even blood sugar control disease diabetes. In general, blood sugar levels are regulated by peptide hormones released by specialist cells termed β-cells.
Apart from insulin, healthy β-cells also release amylin, a peptide hormone that helps minimize sudden increases in blood sugar level after eating by slowing down the emptying of the stomach.
However, amylin is likely to form misfolded clumps, specifically when copper ions are present, which damage β-cells and lead to type II diabetes.
According to Mariusz Jaremko, a KAUST researcher, metal ions can also offset the aggregation of peptides under certain conditions. Jaremko headed the study in collaboration with scientists from the University of Wroclaw in Poland.
For an in-depth investigation of the process, the researchers are studying the interaction of copper(II) ions with amylin and its molecular analogs.
Such knowledge would give us insights into the molecular mechanisms of type II diabetes, enabling us to design new strategies and therapies against this disease.”
Mariusz Jaremko, Researcher, KAUST
As part of their recent study, the researchers analyzed the impact of copper ions on the aggregation of two analogs of human amylin—an amylin-simulating drug named pramlintide and amylin from rats.
We found that differences in the structures of pramlintide and rat amylin mean that copper ions impede the aggregation of pramlintide, but not rat amylin.”
Mawadda Alghrably, Researcher, KAUST
Alghrably is a Ph.D. Student in Jaremko’s group.
The group analyzed the process with the help of various techniques, such as nuclear magnetic resonance (along with Abdul-Hamid Emwas from KAUST CoreLabs), and a “thioflavin T” fluorescence assay of protein aggregation.
In the study, although both amylin analogs were found to bind copper, pramlintide could bind it in two distinct ways, thanks to an additional copper-binding histidine amino acid present in pramlintide but not rat amylin.
The binding of copper ion to this histidine perhaps accounts for why copper minimized the aggregation of pramlintide but not the aggregation of rat amylin, concluded the group.
According to Alghrably, The researchers are continuing to decrypt the molecular basis of amylin aggregation.
Understanding how these molecules behave, could ultimately help to facilitate the design of new efficient drugs and therapies for type II diabetes.”
Mawadda Alghrably, Researcher, KAUST
Source:
Journal reference:
Alghrably, M., et al. (2020) Copper(II) and Amylin Analogues: A Complicated Relationship. The Journal of Organic Chemistry. doi.org/10.1021/acs.inorgchem.9b03498.