In the human body, the inner or outer boundaries of organs are lined with the supposed epithelial sheets.
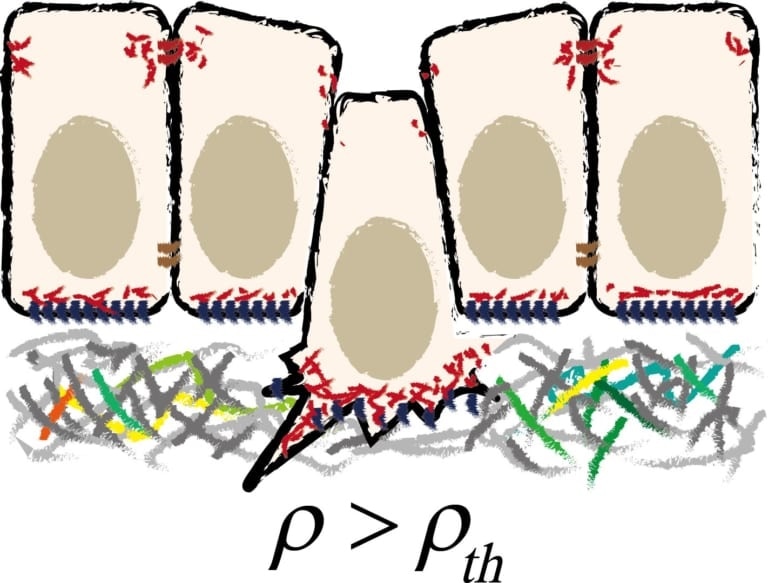
Schematic illustration of cell extrusion from epithelial tissue. Image Credit: Kanazawa University.
These are epithelial cell layers that can separately modify their three-dimensional (3D) shape—which is what occurs during biological processes, like cancer formation (carcinogenesis), physiological equilibrium (homeostasis), or organ development (morphogenesis).
Of specific interest is the cell extrusion process, in which a single cell loses its “bottom” or “top” surface and later pushed out of the layer. There has been a lack of comprehensive insight into this phenomenon from a mechanical perspective, but recently, Satoru Okuda and Koichi Fujimoto both from Kanazawa University have found that there is a purely mechanical cause for the extrusion of cells.
From the mechanical standpoint, a simple (single-layer) epithelial sheet is similar to foam and can be denoted as an interconnected polyhedral layer. Such a foam model was used by Fujimoto and Okuda to explain a monolayer of epithelial cells, with every cell being a polyhedron with an average volume V.
Each cell is additionally defined by the number of adjacent cells n, the area of the basal (“bottom”), and the area of the apical (“top”) surface.
The model, considering the mechanical forces between adjacent cells, results in a formula for the overall mechanical energy of an epithelial sheet as a function of just a few parameters, including n and V, and also the in-plane density and a quantity known as sharpness.
This quantity can differentiate between scenarios where basal and/or apical surfaces are either present or absent (a disappeared apical surface suggests basal extrusion and in the following order).
The scientists were able to gain useful insights into the mechanics of an epithelial sheet by analyzing how the energy alters by modifying these few parameters.
The major finding of both Okuda and Fujimoto is that the system shows an innate mechanical instability: slight variations in cell density or cell topology can cause the extrusion of cells without extra forces being applied. Moreover, it turns out that a cell experiencing extrusion creates forces inside the layer, which can guide the extrusion of other cells to both sides of the layer.
The researchers also noticed several agreements between the results of their model and observations made in living systems, like the existence of different epithelial geometries (for example, pseudostratified structures or “rosette”).
The new model certainly has limitations, for instance, the belief that the entire sheet, as well as the individual cell surfaces, is flat and not curved. But according to the scientists, “despite its limitations, [the] model provides a guide to understanding the wide range of epithelial physiology that occurs in morphogenesis, homeostasis, and carcinogenesis.
Source:
Journal reference:
Okuda, S & Fujimoto, K (2020) A Mechanical Instability in Planar Epithelial Monolayers Leads to Cell Extrusion. Biophysical Journal. doi.org/10.1016/j.bpj.2020.03.028.