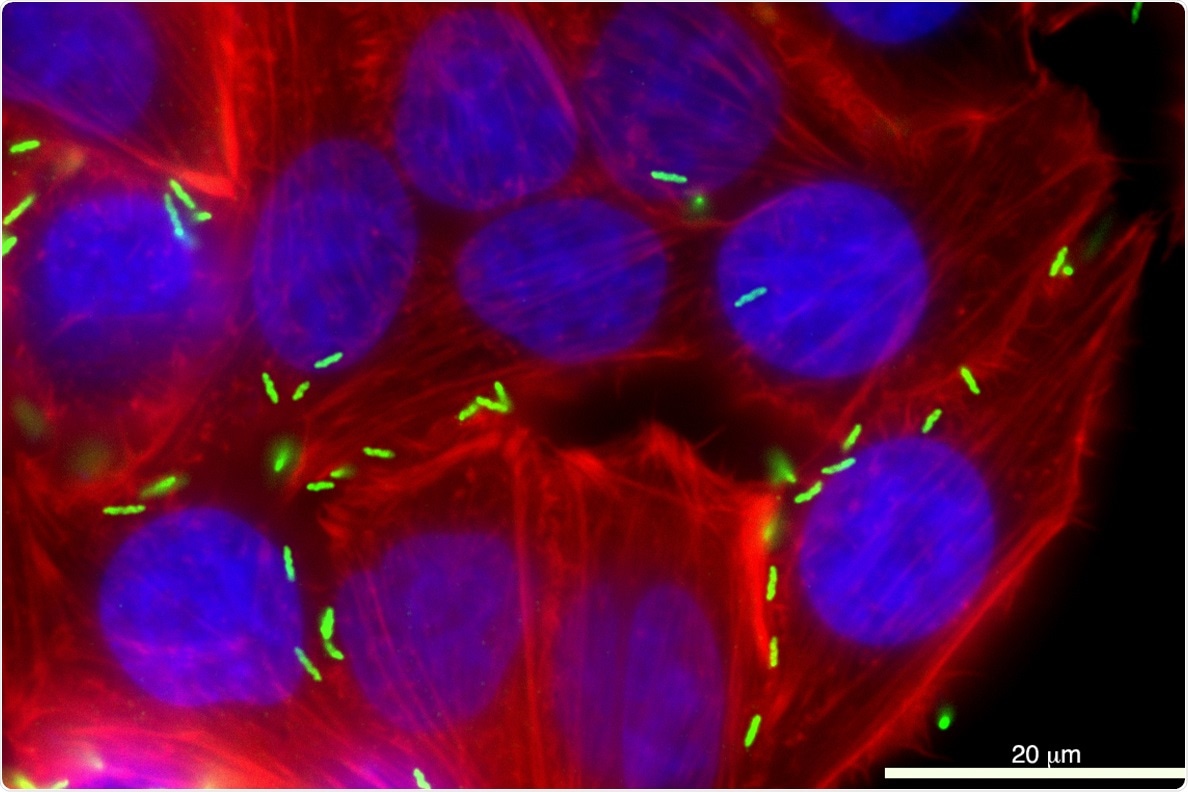
Campylobacter jejuni (green) is shown infecting a human cell. The bacteria account for 400 to 500 million cases of diarrhea annually. Image Credit: Washington State University.
If a person has consumed cross-contaminated food or undercooked poultry by cleaning raw chicken, he or she is more likely to be familiar with the food-borne pathogen.
Negretti is a lead member of the research team in Michael Konkel’s Laboratory.
A new study based on the research work recently published in the Nature Communications journal has reported that a secreted protein, called CiaD, enables Campylobacter to enter the cells and takes control of significant cell processes by modifying the composition of a protein complex within the cell.
By offering a better understanding of the infection process and the particular actions of the proteins secreted by Campylobacter, the study gives the WSU researchers and the rest of the community a basis for interpreting why infections take place and persist.
Until the latest finding made by the Konkel Laboratory, the functions of the bacterial proteins and the way they infect the cell continued to be an enigma.
We knew these things were happening, but we didn’t know how, now, if we can stop this process, disease won’t happen.”
Nick Negretti (’20 PhD), School of Molecular Biosciences, Washington State University
The study was financially supported by a five-year, $1.9 million grant from the National Institutes of Health and is based on 20 years of study in the Konkel Laboratory.
Campylobacter jejuni, which is largely associated with vomiting, nausea, and bloody diarrhea once ingested, releases proteins that penetrate the cells lining the intestinal tract and this enables it to escape the immune system.
The bacteria are responsible for causing 400 to 500 million cases of diarrhea every year, and because of its antibiotic resistance, the World Health Organization has recognized it as a major threat.
Moreover, the infection is associated with stunted linear growth in impoverished kids, and a higher incidence of Guillain-Barré syndrome in developed nations. In the Guillain-Barré syndrome, the nerves are attacked by the body’s immune system.
The study was a seven-year joint effort, which involved the use of advanced molecular biology and biochemistry techniques.
The research work was performed in association with scientists Geremy Clair and Joshua Adkins from the Pacific Northwest National Laboratory. Through mass spectroscopy, Adkins and Clair were able to analyze protein-to-protein interaction that allowed the WSU team to narrow down their focus and reveal the CiaD target.
According to Konkel, the study would not have been possible without the help of post-doctoral fellow Prabhat Talukdar and graduate students, Courtney Klappenbach and Cody Lauritsen, who headed the study via its final stretch amid the ongoing pandemic.
The team is now hoping that the research work will provide real-world solutions, especially in identifying ways to block the pathogen from stunting the growth in children.
With this finding, we can speculate that processes like this that affect the cell could impact the intestinal cell’s ability to form the correct structures to absorb nutrients. While this is a mechanistic level of understanding, the answers to how the bacteria are specifically affecting cells in the body could have broader ranging impacts into understanding the public health importance of this pathogen.”
Nick Negretti (’20 PhD), School of Molecular Biosciences, Washington State University
The researchers are also looking forward to learn the functions of other secreted proteins.
A significant innovation into interpreting the C. jejuni disease was made in 1999 when the Konkel Laboratory found that proteins are released by the bacterium. Then in 2009, the CiaD protein was discovered by Jeffrey Christensen, a post-doctoral fellow in the Konkel Laboratory.
“We then identified CiaD was delivered to the host cells in 2013. A major question for the past 20-years has been: what are these secreted proteins and what do they do? This is just the first protein to have an identified cell target,” Konkel concluded.
Source:
Journal reference:
Negretti, N. M., et al. (2021) The Campylobacter jejuni CiaD effector co-opts the host cell protein IQGAP1 to promote cell entry. Nature Communications. doi.org/10.1038/s41467-021-21579-5.