A new study by researchers at the RIKEN Cluster for Pioneering Research (CPR) in Japan has shown that it is viable to reduce tumor growth by a therapy that involves tagging cancer cells with various therapeutic molecules.
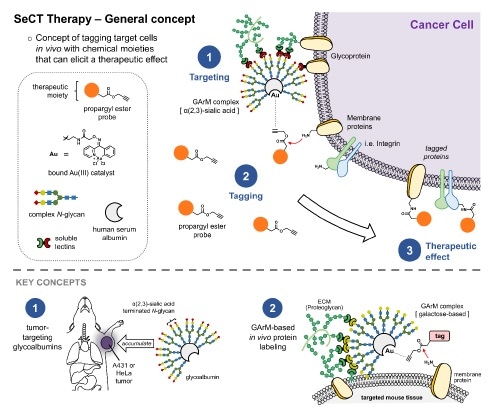
Selective Cell Tagging (SeCT) therapy is a concept based on tagging specific cells in vivo with therapeutic chemical compounds. Here, gold-mediated, propargyl ester-based protein labeling was combined with tumor targeting glycoalbumins to make a glycosylated metalloenzyme that can label tumor cells in mice with drugs that fight cancer. Image Credit: RIKEN.
The study, published in Science Advances on April 23rd, 2021, was headed by Katsunori Tanaka and Kenward Vong from RIKEN CPR.
The researchers could inhibit tumor formation in mice, in one case, by targeting cancer cells using a compound that renders it hard for the cells to clump together and develop tumors. In the case of already existing tumors, cancer cells were targeted using toxic compounds that killed them.
Existing treatments for cancer present a major problem that their effects are not restricted to cancerous cells in the body. Chemotherapy’s side effects are prominent—exhaustion, hair loss, nerve damage, nausea, and compromised immune system.
The dream of achieving the ability to target particularly cancer cells—and only cancer cells—using therapeutic compounds is gradually turning into a reality, and the new research by Tanaka’s team from RIKEN CPR is the proof-of-principle.
We have succeeded for the first time in treating cancer using metal-catalyzed chemistry in mice.”
Katsunori Tanaka, RIKEN Cluster for Pioneering Research
The process is based on the previous study by the researchers that involves using artificial gold-based enzymes—commonly referred to as metalloenzymes—for tagging proteins within the body. The metalloenzyme and the tagging agent are both injected into the body, but separately.
The metalloenzyme is designed to be glycosylated, that is, it includes sugar chains named glycans bound to its surface. Particular glycans are selected so that they can attach to the target cells within the body. For example, it is possible to identify various cancer cells by using the exclusive types of lectins, or glycan-binding proteins, embedded in their outer membranes.
In the case of this experiment, the team designed a glycosylated metalloenzyme that would be able to bind itself to the particular lectins that exist outside of HeLa cancer cells, thereby targeting them. Once the tagging agent reacts with the metalloenzyme, it can carry out the preferred function and tag the protein of interest on the cancer cell. Thus, only cancer cells targeted by the glycosylated metalloenzyme are tagged.
The researchers conducted two crucial targeted drug-delivery tests. The first test involved the use of a kind of RGD that turned functional once it reacted with the artificial enzyme attached to the target cancer cell. RGD was selected as the previous testing showed that it interferes with the potential of cancer cells to clump together and develop tumors.
HeLa cancer cells were injected into mice, following which both the RGD and glycosylated metalloenzyme were also injected. The artificial enzyme alone, RGD alone, or saline was injected into control mice. The mice were monitored for 81 days.
All the control mice were found to develop tumors and died well before 81 days. By contrast, the mice treated with selective cell therapy and RGD tagging exhibited a survival rate of 40%. Imaging analysis showed that the onset and progression of tumors were interrupted by the treatment.
The second test was built to attack already formed tumors. To this end, the researchers used the same glycosylated metalloenzyme, however with a kind of non-toxic doxorubicin that turned functional once it reacted with the metalloenzyme.
Previous testing indicated that the agent was innocuous until it reacted with the metalloenzyme. As soon as it reacts, it releases toxic doxorubicin. Thus, the drug affected only targeted cancer cells.
Testing in mice was analogous to testing with RGD, apart from the fact that tumor formation was allowed for a week before the artificial enzyme and tagging agent were injected. Mice that received the real treatment exhibited reduced tumor growth and an increased survival rate for 77 days.
We were able to use our system to carry metalloenzymes to cancer cells in living mice, which reacted with tagging agents to deliver targeted drug therapies that reduced tumor onset and growth. The next step is certainly clinical application in humans.”
Katsunori Tanaka, RIKEN Cluster for Pioneering Research
Source:
Journal reference:
Vong, K., et al. (2021) Disrupting tumor onset and growth via selective cell tagging (SeCT) therapy. Science Advances. doi.org/10.1126/sciadv.abg4038.