A recent study shows that a critical intermediate in normal cellular metabolism is also a cause for cell death in the right sense. The study was performed by Wanli Liu and Yonghui Zhang of Tsinghua University, and Yong Zhang of Peking University in Beijing.
Visual representation of the project. Image Credit: Wanli Liu.
The findings of the study, which was published on April 26th, 2021, in the open-access journal PLOS Biology, could lead to better insights into the harm caused by strokes, as well as provide a potential drug target for reducing the harm.
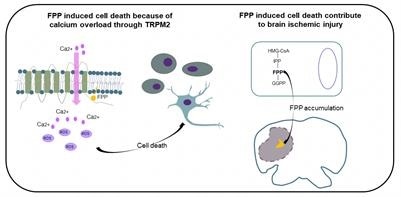
In the mevalonate pathway—a set of biochemical reactions that occurs in each cell and leads to energy production, cell membrane construction, and protein synthesis—farnesyl pyrophosphate (FPP) forms as an intermediate.
While looking for immune cell function regulators, the researchers accidentally found that FPP induced extensive and quick cell death if it occurs in high concentrations outside the cells.
FPP has a highly charged phosphate head and a long hydrophobic hydrocarbon tail, and the research team demonstrated that both are needed for the effect by modifying each in turn. This implies that FPP may interact particularly with certain complementary receptors.
Extracellular calcium loss avoided the lethal effect of FPP, giving another hint regarding the mechanism. The researchers knocked out a number of cation channels and discovered that one, known as TRPM2, led to FPP-induced cell death at a particular level and that an enzyme that blocks FPP-induced TRPM2 open could prevent FPP-induced cell death.
In general, FPP occurs in the microenvironment at too low a concentration to cause cell death. However, this scenario can change during an ischemic stroke because the mevalonate pathway is known to be extremely active in neurons, and neurons may quickly release their cellular contents in stress-induced necrosis, resulting in elevated levels of several biomolecules in the microenvironment that are otherwise rare.
In a mouse model of ischemic injury, the researchers demonstrated that the concentration of FPP increased and that pre-administration of the calcium channel blocker could decrease the degree of injury. Furthermore, inhibitors that block the metabolic development of FPP decreased the severity of the injury as well.
These findings indicate that inhibiting FPP’s activity, either by targeting its metabolic synthesizing pathway or by suppressing TRPM2 to minimize calcium influx, could be a potential way for reducing stroke-related harm.
Far more needs to be understood about this new cell death pathway, including the duration of the timeframe within which such treatments might be therapeutic.
However, Liu and collaborators added that “These findings point to novel, potentially druggable targets to treat ischemic injury. In view of the complex nature of the human ischemic injury, targeting this pathway might best be combined with current therapies to improve the therapeutic effects.”
Source:
Journal reference:
Chen, J., et al. (2021) Farnesyl pyrophosphate is a new danger signal inducing acute cell death. PLOS Biology. doi.org/10.1371/journal.pbio.3001134.