At The University of Texas MD Anderson Cancer Center, scientists have come up with an innovative function for the metabolic enzyme medium-chain acyl-CoA dehydrogenase (MCAD) in glioblastoma (GBM).
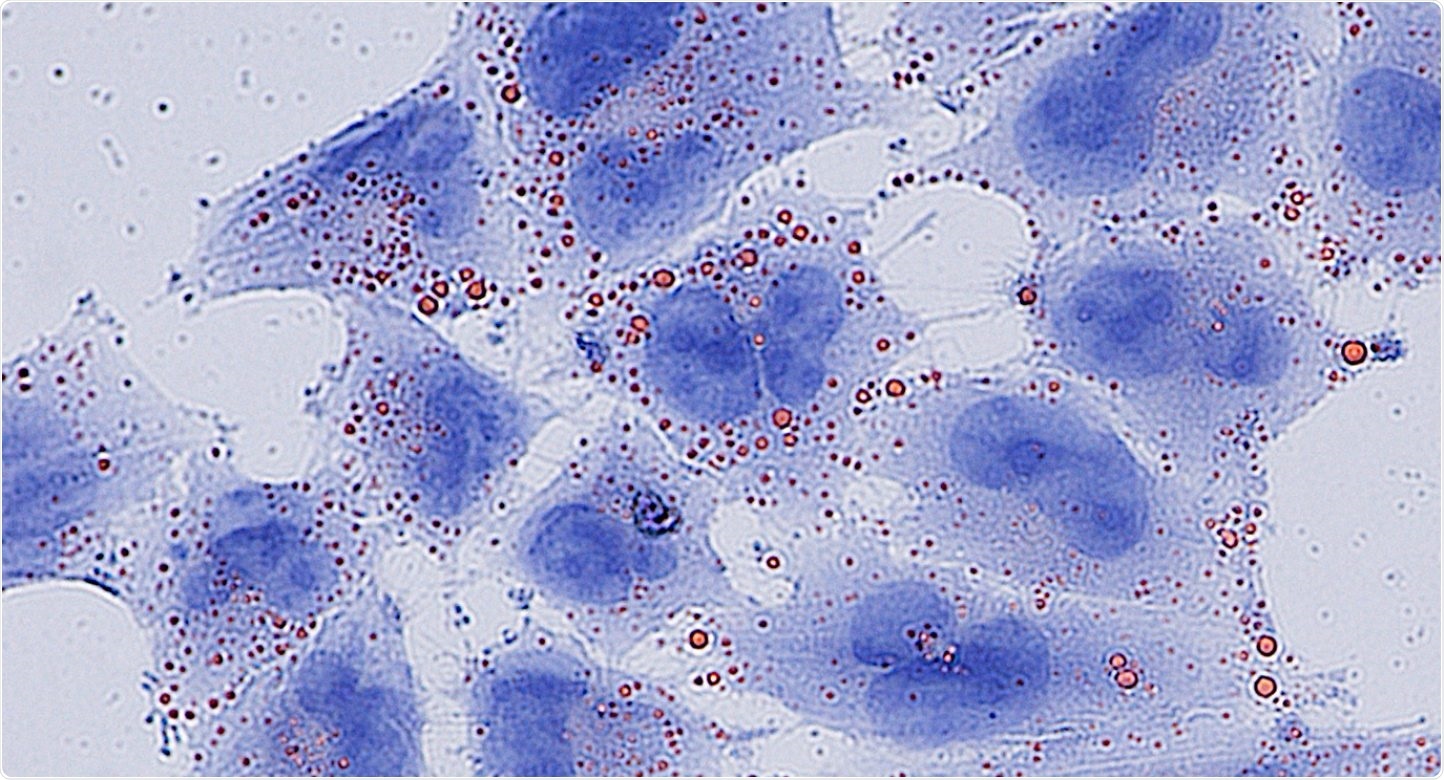
Fatty acids (red) accumulate to toxic levels in glioblastoma cells (blue) without the MCAD enzyme. Image Credit: Francesca Puca, PhD.
MCAD inhibits the accumulation of toxic lipids apart from its normal role in energy production. Thus, targeting MCAD leads to irreversible damage and cell death particularly in cancer cells.
The study was recently published in Cancer Discovery, a journal of the American Association for Cancer Research. Preclinical results unravel a significant new understanding of metabolism in GBM and support the advent of MCAD inhibitors as an innovative treatment approach. At present, the researchers are working to create targeted therapies against the enzyme.
With altered metabolism being a key feature of glioblastoma, we wanted to better understand these processes and identify therapeutic targets that could have real impact for patients. We discovered that glioblastoma cells rely on MCAD to detoxify and protect themselves from the accumulation of toxic byproducts of fatty acid metabolism. Inhibiting MCAD appears to be both potent and specific in killing glioblastoma cells.”
Francesca Puca, PhD, Study Lead Author and Instructor of Genomic Medicine, MD Anderson Cancer Center
The researchers unraveled metabolic genes key to GBM survival by performing a functional genomic screen in a special preclinical model system that allowed an in vivo study with patient-derived GBM cells. The team analyzed 330 metabolism genes in this model and found that various enzymes involved in fatty acid metabolism were crucial for GBM cells.
The researchers focused on MCAD since it was found in several GBM models and identified at high levels in GBM cells with respect to normal brain tissue. Detailed studies found that inhibiting MCAD in GBM cells led to severe mitochondrial failure due to the toxic accumulation of fatty acids, which are usually degraded by MCAD.
This led to an irreversible and catastrophic cascade of events from which GBM cells could not recover, noted Andrea Viale, MD, senior author of the study and assistant professor of Genomic Medicine.
It appears that the downregulation of this enzyme triggers a series of events that are irreversible, and the cells are poisoned from the inside. Usually, tumor cells are able to adapt to treatments over time, but, based on our observations, we think it would be very difficult for these cells to develop resistance to MCAD depletion.”
Andrea Viale, Study Senior Author and Assistant Professor of Genomic Medicine, MD Anderson Cancer Center
Although inhibition of MCAD seems to be harmful to the survival of GBM cells, the researchers repeatedly identified that normal cells in the brain were not impacted by the loss of the enzyme. This indicates that targeting MCAD could be selective in destroying only cancer cells. This observation is supported by the fact that children and animals born with an MCAD deficiency can live normally with a modified diet.
It has become clear that MCAD is a key vulnerability unique to glioblastoma, providing us a novel therapeutic window that may eliminate cancer cells while sparing normal cells. We are looking for discoveries that will have significant benefits to our patients, and so we are encouraged by the potential of these findings. We are actively working to develop targeted therapies that we hope will one day provide an effective option for patients.”
Giulio Draetta, MD, PhD, Study Senior Author, Chief Scientific Officer and Professor of Genomic Medicine, MD Anderson Cancer Center
The researchers have characterized the three-dimensional structure of the MCAD protein in a complex with innovative small molecules engineered to inhibit the activity of the enzyme. With the discovery of potential drug candidates, the team will collaborate with MD Anderson’s Therapeutics Discovery division to analyze these drugs and optimize them toward clinical trials.
Source:
Journal reference:
Puca, F., et al. (2021) Medium-chain acyl CoA dehydrogenase protects mitochondria from lipid peroxidation in glioblastoma. Cancer Discovery. doi.org/10.1158/2159-8290.CD-20-1437.