New research indicates that new brain cells are continuously formed in response to physical exercise, injury, and mental stimulation.
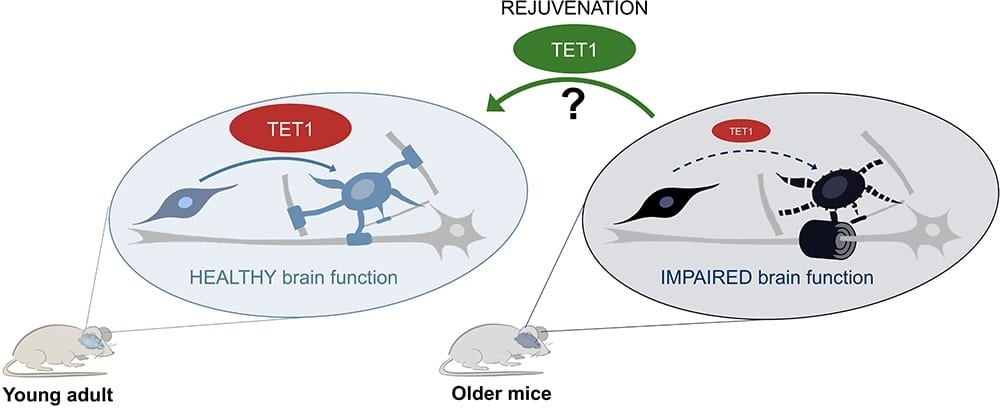
In young adult mice (left), TET1 is active in oligodendroglial cells especially after injury and this leads to new myelin formation and healthy brain function. In old mice (right), the age-related decline of TET1 levels impairs the ability of oligodendroglial cells to form functional new myelin. The authors are currently investigating whether increasing TET1 levels in older mice could rejuvenate the oligodendroglial cells and restore their regenerative functions. Image Credit: Advanced Science Research Center.
Glial cells, particularly the ones known as oligodendrocyte progenitors, are extremely responsive to external signals and injuries. These cells can identify changes in the nervous system and subsequently form new myelin. The newly formed myelin envelopes the nerves and offers metabolic support and precise transmission of electrical signals.
But as people grow older, less myelin is formed in reaction to external signals and this continuous decline has been associated with age-related motor and cognitive deficits identified in older individuals in the general population.
People with neurodegenerative diseases, such as Alzheimer’s disease or multiple sclerosis develop impaired myelin formation. This impairment has been identified as one of the leading causes of progressive clinical deterioration in these patients.
A research work performed by the Neuroscience Initiative team at the Advanced Science Research Centre at The Graduate Center, CUNY (CUNY ASRC) has detected a molecule known as ten-eleven-translocation 1 (TET1), as a crucial component in myelin repair. The study shows that TET1 alters the DNA in specific glial cells in adult brains. This can induce the formation of new myelin in response to injuries. The study has been published in the Nature Communication journal.
We designed experiments to identify molecules that could affect brain rejuvenation. We found that TET1 levels progressively decline in older mice, and with that, DNA can no longer be properly modified to guarantee the formation of functional myelin.”
Sarah Moyon, PhD, Study Lead Author and Research Assistant, CUNY ASRC Neuroscience Initiative
The authors combined whole-genome sequencing bioinformatics and demonstrated that DNA modification caused by TET1 in young adult mice was crucial to promote healthy communication among cells in the central nervous system and ensure proper function.
The researchers also demonstrated that young adult mice with a genetically modified TET1 in the myelin-forming glial cells were incapable of producing functional myelin and thus their behavior was similar to older mice.
This newly identified age-related decline in TET1 may account for the inability of older individuals to form new myelin. I believe that studying the effect of aging in glial cells in normal conditions and in individuals with neurodegenerative diseases will ultimately help us design better therapeutic strategies to slow the progression of devastating diseases like multiple sclerosis and Alzheimer’s.”
Patrizia Casaccia, Study Primary Investigator and Founding Director, CUNY ASRC Neuroscience Initiative
Casaccia is also a professor of Biology and Biochemistry at The Graduate Center, CUNY.
The findings could also hold major implications for molecular rejuvenation of aging brains in healthy people, added the researchers. Upcoming studies focused on boosting the levels of TET1 in older mice to ongoing to define if the molecule could save the formation of new myelin and favor proper neuro-glial communication. The long-term goal of the research team is to encourage the recovery of motor and cognitive functions in older individuals as well as in patients with neurodegenerative disorders.
Source:
Journal reference:
Moyon, S., et al. (2021) TET1-mediated DNA hydroxymethylation regulates adult remyelination in mice. Nature Communications. doi.org/10.1038/s41467-021-23735-3.