Cancer, a devastating disease, is the second predominant cause of death in the United States. One of the characteristics of cancer is genomic instability, or the tendency to accumulate mutations and cause damage to the DNA leading to genome alterations at the time of cell division.
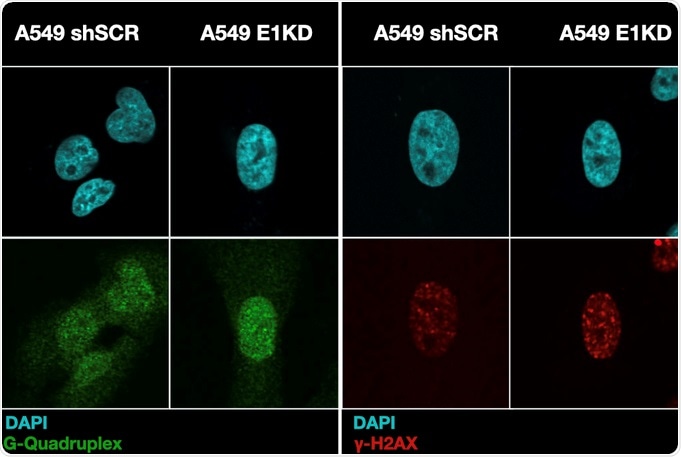
G-Quadruplexes (green) and the histone marker gamm-H2AX (red) localize to the nucleus in cells with or without hnRNP E1. Image Credit: Medical University of South Carolina—Dr Bidyut Mohanty and Joseph Karam.
DNA mutations can occur due to exposure to X-ray radiation or ultraviolet or from some chemicals called carcinogens. Human cells have perfected processes to monitor and repair damaged DNA.
Genome stability can also be threatened by the translation of specific messenger RNAs (mRNA). mRNA, mimicked from DNA, functions as the genetic code for the building of proteins. Some mRNAs are known to be linked with cancer metastasis. To undermine this threat, a specific protein, heterogeneous nuclear ribonucleoprotein E1 (hnRNP E1), attaches to these mRNAs and restrains them from producing proteins.
Scientists from the Medical University of South Carolina earlier displayed how hnRNP E1 attaches to metastatic-linked RNAs to prevent their translation. hnRNP E1 attaches RNA in the cell cytoplasm; however, the protein is also seen in the cell’s nucleus. This directed the scientists to speculate that hnRNP E1 might also interact with DNA.
The observations were published on July 16th, 2021, in the Life Science Alliance journal. The results elaborate a new role for hnRNP E1 in attaching DNA in the nucleus.
We found that this RNA binding protein not only has broad RNA binding function, but that it also binds to similar sequences on the DNA. The protein binds DNA in a sequence- and structure-specific manner to maintain genome integrity and sense or prevent DNA damage.”
Bidyut K. Mohanty, PhD, Study Lead Author and Assistant Professor, College of Medicine, Medical University of South Carolina
The binding and interaction of hnRNP E1 with RNA is widely researched; however, Mohanty’s observation that hnRNP E1 also attaches DNA has unlocked new research avenues to investigate. hnRNP E1’s DNA binding is not restricted to a few sites, but the protein has abundant possible binding sites on the genome, allowing it to sense or inhibit DNA damage across the genome.
The researchers also identified that hnRNP E1 attaches to a specific structure that can develop on DNA called an I-motif. I-motifs form in regions enriched in the nucleotide cytosine and function as regulators of gene expression. As DNA is made of specific bonds between nucleotides, called base-pairings, many guanine bases are observed opposite of the cytosine-rich I-motifs.
These guanine-rich regions can form their structure called G-quadruplexes (G4). G4s are found at the start of several oncogenes (genes that play a role in the formation of tumor cells). However, it is not yet clear if I-motifs and G4s can prevail at the same time or if they are mutually exclusive. Thus, hnRNP attaching to I-motif regions may suppress the formation of G4 structures to safeguard the cell.
Mohanty hypothesized that hnRNP E1 would safeguard against genomic instability by suppressing G4s and retaining I-motifs. Examinations employing cells that do not possess hnRNP E1 showed a decrease in I-motifs while at the same time they demonstrated increases in G4s, mutations, and DNA damage signals.
Treating these cells with additional DNA damaging agents, like UV and hydroxyurea (a carcinogen), resulted in an intensified DNA damage response from the cells which directed them to halt progressing through the cell cycle.
This protein, involved in prevention of metastasis, may also have a role as a DNA damage sensing protein. This is a great launching point for future studies.”
Joseph Karam, Study Second Author and Graduate Student, Biochemistry Department, Medical University of South Carolina
The observations have significant relevance to the field of cancer biology and genetics. For many years, scientists have been analyzing the contribution of G4s to cancer biology. The association with oncogenes makes these regions the target for anticancer therapies and drug design.
Insight on the protein-DNA interactions that take place at the sites opposite G4s can contribute to the effectiveness of these drugs, thus enabling better drug targeting and specificity.
Source:
Journal reference:
Mohanty, B. K., et al. (2021) Heterogeneous nuclear ribonucleoprotein E1 binds polycytosine DNA and monitors genome integrity. Life Science Alliance. doi.org/10.26508/lsa.202000995.