Scientists from the Centre for Genomic Regulation (CRG) and Pulmobiotics S.L have developed the first “living medicine” to treat antibiotic-resistant bacteria thriving on medical implants’ surfaces. The scientists developed the treatment by eliminating a common bacteria’s capability to induce disease and repurposing it to combat harmful microbes.
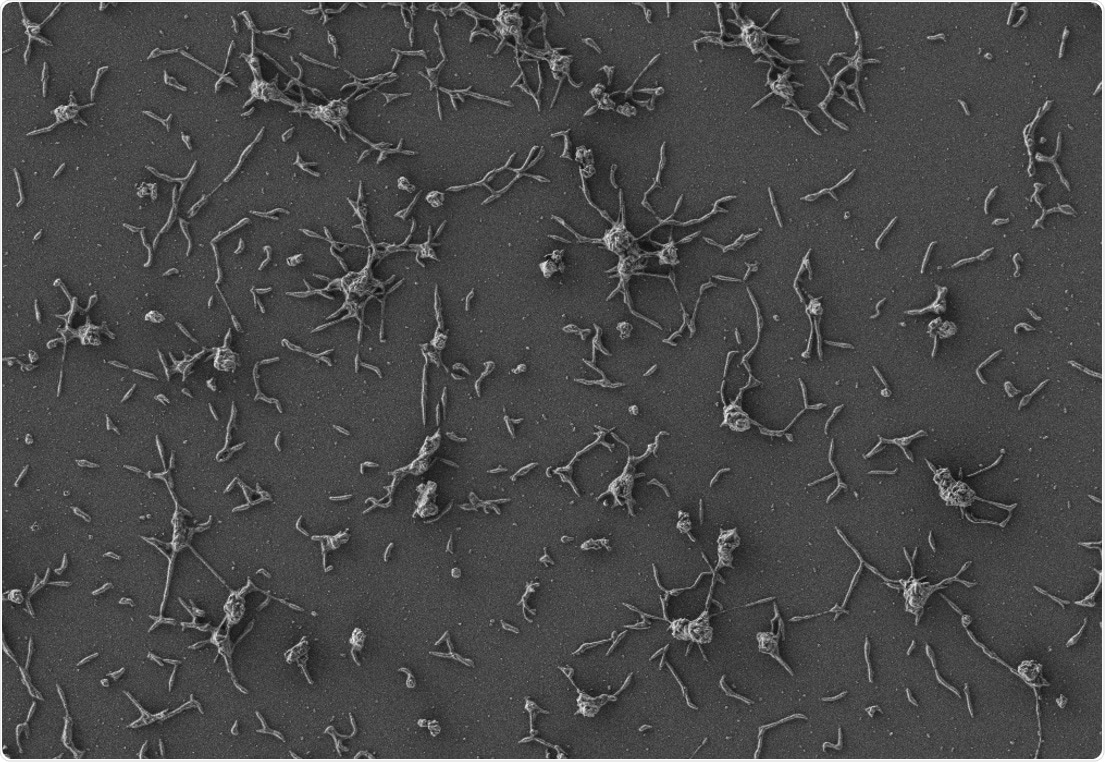
Scanning electron microscope image of Mycoplasma pneumoniae cells, small bacteria that are naturally adapted to the human lung. Image Credit: María Lluch/Center for Genomic Regulation.
The experimental treatment was examined on infected catheters in vivo, ex vivo, and in vitro, effectively treating infections on the three testing methods employed. The researchers state that while the therapy was injected below the skin of mice, they observed that it treated infections in 82% of the animals dealt with.
The observations are a vital step for the generation of novel treatments for infections affecting medical implants like pacemakers, catheters, and prosthetic joints. These are greatly resistant to antibiotics and account for 80% of all infections attained in hospital settings.
The current research was supported by the “la Caixa” Foundation through the CaixaResearch Health call, the European Research Council (ERC), the MycoSynVac project under the EU’s Horizon 2020 research and innovation program, the Generalitat de Catalunya, and the Instituto de Salud Carlos III. The research was published on October 6th, 2021, in the Molecular Systems Biology journal.
The novel treatment particularly targets biofilms—colonies of bacterial cells that combine on a surface. Medical implant surfaces are perfect growing conditions for biofilms. The biofilms form impenetrable structures that hinder the antibiotics or the human immune system from annihilating the bacteria found within.
Biofilm-linked bacteria are anticipated to be highly resistant—more than a thousand times—to antibiotics when compared to free-floating bacteria.
The widely common species of biofilm-linked bacteria is Staphylococcus aureus. S. aureus infections normally require patients to surgically take away any infected medical implants as they do not respond to traditional antibiotics. Antibodies or enzymes can be employed as alternative therapies, however as they are broad-spectrum treatments, they are highly toxic for normal cells and tissues, resulting in unwanted side effects.
The researchers hypothesized that introducing living organisms that directly synthesize enzymes in the local vicinity of biofilms is an inexpensive and secure way of treating infections. The perfect vectors are bacteria-their small genomes that can be altered employing simple genetic manipulation.
The scientists picked up Mycoplasma pneumoniae—a common species of bacteria lacking a cell wall—to engineer. The lack of a cell wall makes it easier to discharge the therapeutic molecules that combat infection and also helps it in avoiding human immune system detection.
There also exist few other benefits of employing M. pneumoniae as a vector—lowered risk of mutating new capabilities, and incompetence to transfer its altered genes to other microbes thriving nearby.
M. pneumoniae was initially altered so that it cannot induce illness. Further tweaks were made so that it secretes two various enzymes that dissolve biofilms and attacks the cell walls of the bacteria found within. The scientists also altered the bacteria so that it produces antimicrobial enzymes more effectively.
M. pneumoniae is naturally adapted to the lung and hence the scientists initially aimed to employ the altered bacteria to treat biofilms developing around breathing tubes.
Our technology, based on synthetic biology and live biotherapeutics, has been designed to meet all safety and efficacy standards for application in the lung, with respiratory diseases being one of the first targets. Our next challenge is to address high-scale production and manufacturing, and we expect to start clinical trials in 2023.”
María Lluch, Study Co-Corresponding Author and Chief Science Officer, Pulmobiotics
The altered bacteria might also have lasting applications for other diseases.
Bacteria are ideal vehicles for ‘living medicine’ because they can carry any given therapeutic protein to treat the source of a disease. One of the great benefits of the technology is that once they reach their destination, bacterial vectors offer continuous and localized production of the therapeutic molecule.”
Luis Serrano, Study Co-Author and Director, Center for Genomic Regulation
Luis Serrano further adds, “Like any vehicle, our bacteria can be modified with different payloads that target different diseases, with potentially more applications in the future.”
Luis Serrano is also a Research Professor at ICREA.
Source:
Journal reference:
Garrido, V., et al. (2021) Engineering a genome-reduced bacterium to eliminate Staphylococcus aureus biofilms in vivo. Molecular Systems Biology. doi.org/10.15252/msb.202010145.