Reviewed by Danielle Ellis, B.Sc.Jun 6 2022
Ubiquitin is a tiny regulatory protein that can be present in most eukaryotic cells. It is important for changing the function of other proteins, and it is especially important for protein breakdown and localization inside the cell. Ubiquitin regulates these actions through ubiquitination (attaching to and detaching from a target protein).
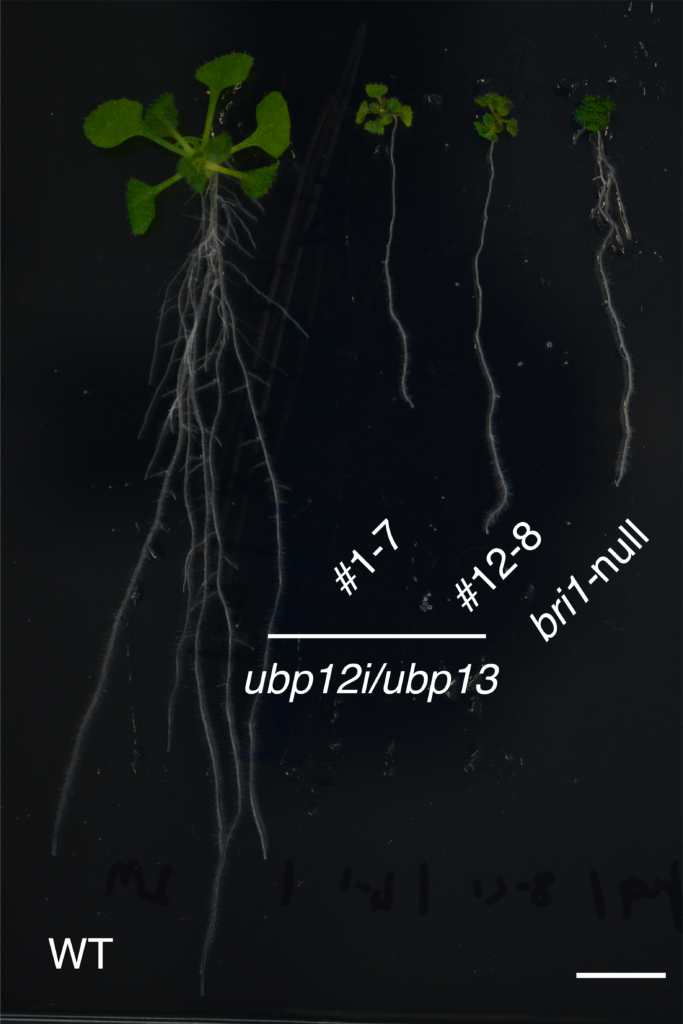 Compared to a wild type Arabidopsis (WT, left), plants that lack either UBP12 and UBP13 (#1-7 and #12-8, center) or BRI1 (bri1-null, right) have extremely stunted growth. Image Credit: Yongming Luo, et al. EMBO Reports.
Compared to a wild type Arabidopsis (WT, left), plants that lack either UBP12 and UBP13 (#1-7 and #12-8, center) or BRI1 (bri1-null, right) have extremely stunted growth. Image Credit: Yongming Luo, et al. EMBO Reports.
Associate Professor Takeo Sato of Hokkaido University led a team of scientists from Japan, Belgium, and the United States to find the first examples of deubiquitinating enzymes in plants that operate on proteins in the cell membrane. Their results, which were published in the journal EMBO Reports, explain how these proteins work.
Ubiquitin ligases and deubiquitinating enzymes catalyze the addition of ubiquitin to target proteins, and deubiquitinating enzymes (DUBs) catalyze the elimination of ubiquitin from target proteins. It was unknown which plant DUBs could remove ubiquitin from membrane proteins directly. By this gap in understanding, the regulation of membrane protein stability remained a mystery.
The researchers discovered that two Arabidopsis thaliana DUBs, UBP12 and UBP13, directly attack the BRI1 plant hormone receptor, which is found on the cell membrane.
Brassinosteroids (BRs), steroidal phytohormones that are necessary for progress and expansion, are detected by BRI1. When BRI1 recognizes BR, it usually activates a pathway that controls gene expression in the nucleus.
Although the regulatory system for how BRI1 abundance is optimized (fine-tuned) in the cells is unknown, the amount of cellular BRI1 is critical for correctly mediating the BR signal. UBP12 and UBP13 de-ubiquitinated and stabilized BRI1, according to the researchers.
They demonstrated that Arabidopsis thaliana plants lacking the ability to articulate UBP12 and UBP13 were undersized and significantly less sensitive to BRs in their trials. Growth deficits were largely corrected when a mutant BRI1 (which could not be ubiquitinated) was inserted into these UBP12 and UBP13-deficient plants. UBP12 and UBP13 specifically target and act on ubiquitinated BRI1.
The interaction of UBP12 and UBP13 with BRI1 was found to be a critical regulator of plant development in the study. The findings also revealed how membrane protein stability is sustained—nevertheless, more research is needed to completely comprehend BRI1 dynamics within the cell. Ultimately, the data show that plant DUBs perform similar functions to mammalian DUBs.
Source:
Journal reference:
Luo, Y., et al. (2022) Deubiquitinating enzymes UBP12 and UBP13 stabilize the brassinosteroid receptor BRI1. EMBO Reports. doi.org/10.15252/embr.202153354.