Every year, 5,000 people in the United States are diagnosed with ALS, an incurable neurodegenerative disease that will destroy them within two to five years. A research team led by USC Stem Cell scientist Justin Ichida has discovered two promising avenues for creating new treatments for various forms of this devastating disease, also known as amyotrophic lateral sclerosis or Lou Gehrig’s disease.
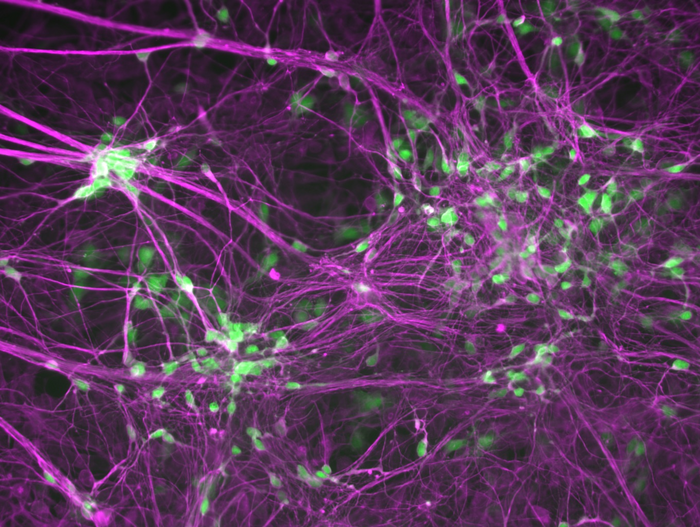 Human induced motor neurons that are labeled with a motor neuron marker HB9 in green and a neuron marker TUJ1 in purple. Image Credit: Ichida Lab.
Human induced motor neurons that are labeled with a motor neuron marker HB9 in green and a neuron marker TUJ1 in purple. Image Credit: Ichida Lab.
The findings were published in two studies, the first in the journal Cell Stem Cell on February 2nd, 2023, and the second in Cell on February 7th, 2023.
A minority of patients have a variety of genetic causes of ALS that can be inherited within families, and a majority have what is known as ‘sporadic’ disease because its causes are unknown. This makes it a difficult challenge to find one treatment that will work for all patients with ALS.”
Gabriel Linares, Study Co-First Author and Postdoc, Keck School of Medicine of USC
Gabriel Linares is a Postdoc in the Ichida lab and a co-first author on both studies.
To address these challenges, the researchers gathered skin and blood samples from patients suffering from both familial and sporadic ALS. The researchers reprogrammed skin and blood cells into motor neurons, which are the nerve cells that control movement and degenerate in disease. These patient-derived motor neurons allowed researchers to screen thousands of FDA-approved drugs and drug-like molecules for those that might be effective against multiple types of ALS.
Linares and Yichen Li, co-first authors of the Cell Stem Cell study, discovered that many of the most effective drugs and drug-like molecules enhanced the activity of androgens, the well-known group of sex hormones that includes testosterone. However, because androgen-boosting drugs may have unwanted or dangerous side effects in ALS patients, the researchers sought to identify a genetic change that might produce similar results.
To do so, they used Connectivity Map, a public bioinformatics database created by the Broad Institute of Harvard and MIT. The scientists accurately guessed that repressing the SYF2 gene would increase the survival of motor neurons derived from patients with various forms of ALS by analyzing this vast database of information about how drugs affect the genetic landscape underpinning diseases.
Furthermore, in mice with ALS, suppressing this gene lowered neurodegeneration, motor dysfunction, and other symptoms.
What’s really exciting is that SYF2 suppression improved symptoms and pathology related to a protein called TDP-43, which can become toxic and is implicated in close to 97% of cases of ALS.”
Yichen Li, Study Co-First Author, Keck School of Medicine of USC
Co-first authors Shu-Ting (Michelle) Hung and Linares describe how hindering a protein, the PIKFYVE kinase, could represent another effective approach for treating several different types of ALS in the second study published in Cell.
The investigators inhibited PIKFYVE in fruit flies, roundworms, mice, and motor neurons derived from patients with various forms of ALS using the drug apilimod, as well as genetic and RNA-based approaches.
They discovered that inhibiting PIKFYVE lowered neurodegeneration, enhanced motor function, and increased life span by stimulating motor neurons to clear toxic proteins via exocytosis, a process in which membrane-bound sacs envelop and actively transport waste to the cell’s exterior.
“We were able to pinpoint precisely how PIKFYVE inhibition mitigates neurodegeneration, which is important for informing the development of new targeted treatments,” stated Hung, a PhD Student in the Ichida Lab.
Ichida, who is the John Douglas French Alzheimer’s Foundation Associate Professor of Stem Cell Biology and Regenerative Medicine at USC, and a New York Stem Cell Foundation–Robertson Investigator, noted, “Our discoveries bring us closer to achieving our big picture goal: finding treatments that can be broadly effective for all patients who suffer from ALS.”
Source:
Journal references:
- Linares, G. R., et al. (2023) SYF2 suppression mitigates neurodegeneration in models of diverse forms of ALS. Cell Stem Cell. doi.org/10.1016/j.stem.2023.01.005.
- Hung, S.-T., et al. (2023) PIKFYVE inhibition mitigates disease in models of diverse forms of ALS. Cell. doi.org/10.1016/j.cell.2023.01.005.