Image Credit: Adisak Riwkratok/shuttershock.com
They also uncovered some of the underappreciated molecular players and events that result in cell differentiation in the gut.
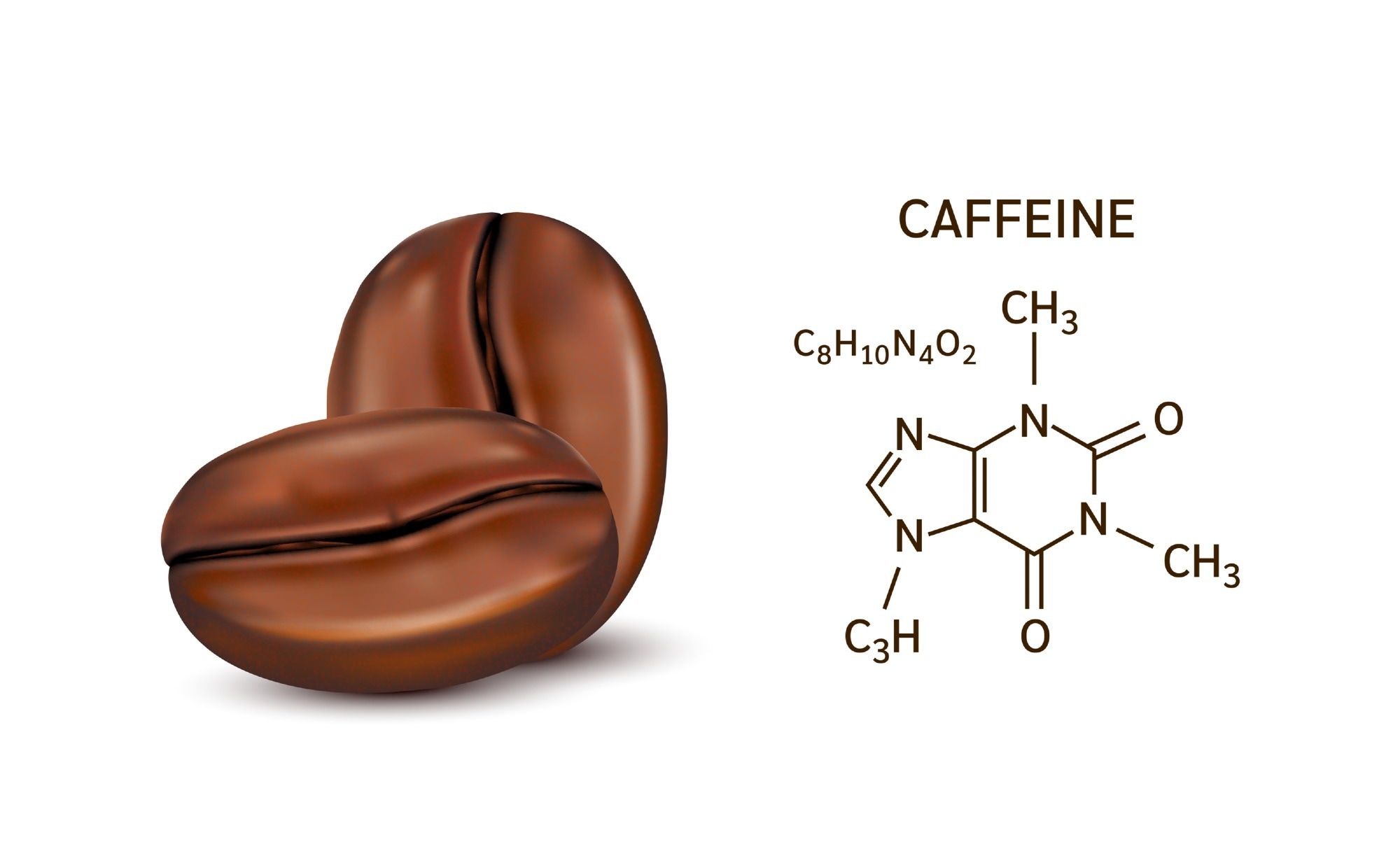
The purine metabolite xanthine, which is present in high concentrations in caffeinated foods, including coffee, tea, and chocolate, is one of these players. Immunity reported the study’s findings.
One of the concepts in our field is that microbes are required for Th17 cell differentiation, but our study suggests that there may be exceptions. We studied the underlying mechanisms of Th17 cell generation in the gut and found some surprising results that may help us to better understand how and why diseases like IBD may develop.”
Jinzhi Duan, Ph.D., Division of Gastroenterology, Hepatology and Endoscopy, Department of Medicine, Brigham and Women’s Hospital
Unexpectedly, the researchers identified a role for xanthine in the gut while highlighting the mechanisms leading to Th17 cell development.
Sometimes in research, we make these serendipitous discoveries—it is not necessarily something you sought out, but it is an interesting finding that opens up further areas of inquiry.”
Richard Blumberg, MD, Study Senior Author and Division of Gastroenterology, Hepatology and Endoscopy, Department of Medicine, Brigham and Women’s Hospital
Blumberg added, “It is too soon to speculate on whether the amount of xanthine in a cup of coffee leads to helpful or harmful effects in a person’s gut, but it gives us interesting leads to follow up on as we pursue ways to generate a protective response and stronger barrier in the intestine.”
Th17 cells, which produce interleukin-17, are considered to be important in the intestine. When a bacterial or fungal infection develops, the cells can send signals that prompt the body to generate additional Th17 cells.
The cells can also assist in constructing a protective barrier in the gut. However, the cells have also been linked to conditions including IBD, psoriasis, rheumatoid arthritis, and multiple sclerosis.
Many mouse models were employed by Duan, co-lead author Juan Matute, MD, Blumberg, and others to explore the molecular mechanisms that result in the formation of Th17 cells. Interestingly, scientists discovered that Th17 cells could multiply even in mice that were devoid of bacteria or mice who had received antibiotics to kill bacteria.
The scientists discovered that even in mice lacking microbes and with genetic signatures indicating cells with protective properties, endoplasmic reticulum stress in intestinal epithelial cells encouraged Th17 cell development through purine metabolites, such as xanthine.
The authors point out that while their research was restricted to cells in the intestine, it is likely that interactions between intestine-specific cells and those in other organs, such as the skin and lung, may have a significant impact on results.
They also point out that their research does not address what causes Th17 cells to become pathogenic or play a role in disease. They emphasize that more research is needed, particularly investigations on human-IBD Th17 cells.
Blumberg added, “While we don’t yet know what is causing pathogenesis, the tools we have developed here may take us a step closer to understanding what causes disease and what could help resolve or prevent it.”
Source:
Journal reference:
Duan, J., et al. (2023). Endoplasmic reticulum stress in the intestinal epithelium initiates purine metabolite synthesis and promotes Th17 cell differentiation in the gut. Immunity. doi.org/10.1016/j.immuni.2023.02.018