In a recent study, scientists used an instrument known as “experimental evolution” under the direction of researchers from the University of Helsinki and the Georgia Institute of Technology.
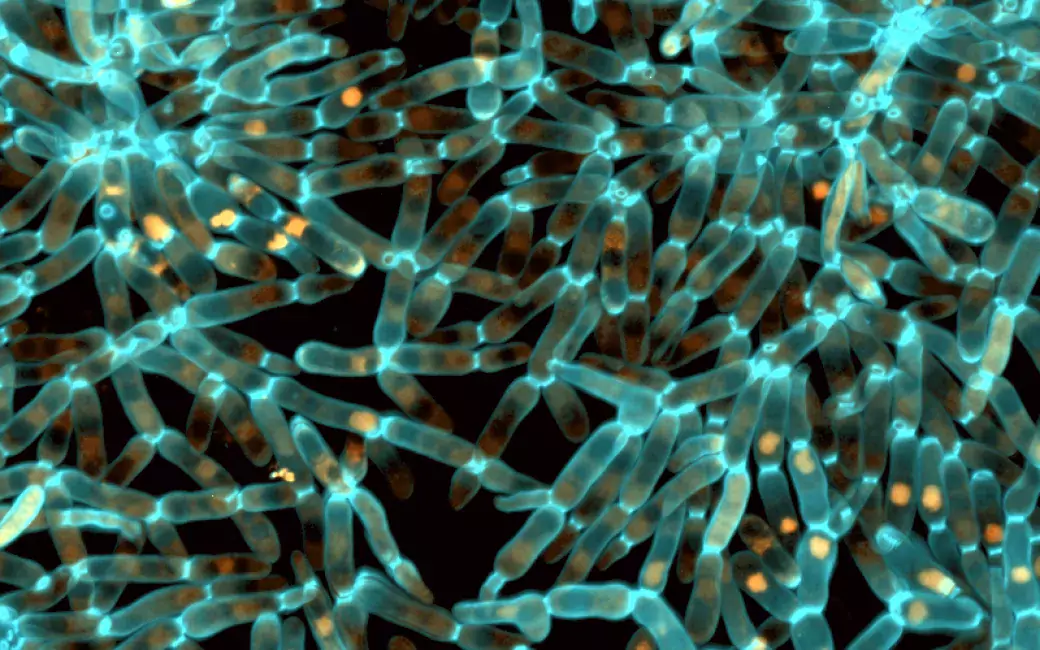 Evolved snowflake yeast. Image Credit: Tony Burnetti
Evolved snowflake yeast. Image Credit: Tony Burnetti
Researchers can study the emergence of novel multicellular functions by studying laboratory yeast that are evolving in the Multicellularity Long Term Evolution Experiment (MuLTEE).
The study highlights the role that protein regulation plays in comprehending evolution.
By demonstrating the effect of protein-level changes in facilitating evolutionary change, this work highlights why knowledge of the genetic code in itself does not provide a full understanding of how organisms acquire adaptive behaviors. Achieving such understanding requires mapping the entire flow of genetic information, extending all the way to the actionable states of proteins that ultimately control the behavior of cells.”
Juha Saarikangas, Associate Professor, Department of Biological and Environmental Sciences, University of Helsinki
Snowflake Yeast Evolves Robust Bodies in 3,000 Generations by Changing Cell Shape
The evolution of robust bodies, or “snowflake yeast,” over 3,000 generations, from weaker than gelatin to as tough and resilient as wood, is one of the most significant multicellular innovations.
According to research findings, this novel multicellular characteristic has a non-genetic basis that functions at the protein folding level. The researchers discovered that when snowflake yeast evolved larger, tougher bodies, the expression of the chaperone protein Hsp90, which aids other proteins in acquiring their functional shape, was gradually turned down.
It turns out that Hsp90 functioned as a crucial tuning knob, causing a central molecule that controls the cell cycle's progression to become unstable and elongating cells as a result. Thus, because of their extended shape, cells can encircle one another to form larger, more mechanically tough multicellular groups.
Hsp90 has long been known to stabilize proteins and help them fold properly, and what we have found is that slight alterations in how Hsp90 operates can have profound effects not just on single cells, but on the very nature of multicellular organisms.”
Kristopher Montrose, Study Lead Author, University of Helsinki
Path to Adaptive Evolution Through Altering Protein Shapes
This work emphasizes the role that non-genetic mechanisms play in quick evolutionary change from an evolutionary standpoint.
We tend to focus on genetic change and were quite surprised to find such large changes in the behavior of chaperone proteins. This underscores how creative and unpredictable evolution can be when finding solutions to new problems, like building a tough body.”
Will Ratcliff, Professor, Georgia Institute of Technology
Source:
Journal reference:
Montrose, K., et al. (2024) Proteostatic tuning underpins the evolution of novel multicellular traits. Science Advances. doi.org/10.1126/sciadv.adn2706